IGEM 2010 Project
We need to start capturing what we are doing, and why.
Please use this page as a starting place.
We need an overall description of our project at the overview level as well as the subsections and phases of implementation.
Math Sub-Projects
Counting Permutations
Improving Oligo Assembler We adapted the lancelator (http://gcat.davidson.edu/IGEM06/oligo.html), a program created by Lance Harden as a part of the 2006 iGem team, so that it would execute faster and can handle longer sequences. The old program could only handle sequences of 300 bp or shorter. Also, the more oligos the program broke the sequence into, the longer it took to run, and for 8 or more the run time was simply unreasonable. However, for the sequences it could handle it always finds the optimal oligos to use. Our program allows the user to enter a sequence of any length, and the length does not significantly effect runtime. In addition, we added extra features such as checking for and removing BioBrick restriction sites and adding BioBrick primers. While our program does not always find the optimal oligos, it still does very well which is an acceptable compromise because the runtime is improved so dramatically.
http://gcat.davidson.edu/iGem10/index.html
Paris IGEM 2007 Results
| T | r |
| 19.5 | 40.16 |
| 20.5 | 39.61 |
| 25.5 | 36.76 |
| 30.5 | 33.78 |
| 35.5 | 30.66 |
| 40.5 | 27.39 |
| 45.5 | 23.97 |
| 50.5 | 20.39 |
| 55.5 | 16.64 |
This is a chart of the experimental group in the Paris IGEM 2007 project. I used the formula r=1-10^(.004*T)/2 where r is the recombination rate (how many times Cre makes a cut and a Scar site is formed) and T is the generation time in minutes (How long it takes the cell to divide).
Paris IGEM 2007 Results
| r | G |
| .05 | 103.3 |
| .10 | 50.3 |
| .15 | 32.6 |
| .20 | 23.7 |
| .25 | 18.4 |
| .30 | 14.9 |
| .35 | 12.3 |
| .40 | 10.4 |
| .45 | 8.9 |
| .50 | 7.6 |
| .55 | 6.6 |
| .60 | 5.8 |
| .65 | 5.0 |
| .70 | 4.4 |
| .75 | 3.8 |
| .80 | 3.3 |
| .85 | 2.8 |
| .90 | 2.3 |
| .95 | 1.8 |
This is a chart of the experimental group in the Paris IGEM 2007 project. I used the formula G=log(1/200)/log(1-r) where r is the recombination rate (how many times Cre makes a cut and a Scar site is formed) and G is the number of generations (or the number of times the cell will need to divide). I am making an assumption here that the initial prey population (the plasmids in the cell) is 200.
Biology Sub-Projects
Codon Optimization
| Segment | Number of Base Pairs | Wild Type Sequence | Optimized Sequence | Deoptimized Sequence |
|---|---|---|---|---|
| 1 | 141 | Atgaaatctaacaatgcgctcatcgtcatcctcggcaccgtcaccctggatgctgtaggcataggcttggttatgccggtactgccgggcctcttgcgggatatcgtccattccgacagcatcgccagtcactatggcgtg | ATGAAATCTAACAACGCGCTGATCGTTATCCTGGGTACCGTTACCCTGGACGCGGTTGGTATCGGTCTGGTTATGCCGGTTCTGCCGGGTCTGCTGCGTGACATCGTTCACTCTGACTCTATCGCGTCTCACTACGGTGTT | ATGAAGAGTAATAATGCCCTAATAGTCATACTAGGAACAGTCACACTAGATGCCGTCGGAATAGGACTCGTCATGCCCGTCCTACCCGGACTACTAAGGGATATAGTCCATAGTGATAGTATAGCCAGTCATTATGGAGTC |
| 2 | 138 | ctatatgcgttgatgcaatttctatgcgcacccgttctcggagcactgtccgaccgctttggccgccgcccagtcctgctcgcttcgctacttggagccactatcgactacgcgatcatggcgaccacacccgtcctg | CTGTACGCGCTGATGCAGTTCCTGTGCGCGCCGGTTCTGGGTGCGCTGTCTGACCGTTTCGGTCGTCGTCCGGTTCTGCTGGCGTCTCTGCTGGGTGCGACCATCGACTACGCGATCATGGCGACCACCCCGGTTCTG | CTATATGCCCTAATGCAATTTCTATGTGCCCCCGTCCTAGGAGCCCTAAGTGATAGGTTTGGAAGGAGGCCCGTCCTACTAGCCAGTCTACTAGGAGCCACAATAGATTATGCCATAATGGCCACAACACCCGTCCTA |
| 3 | 177 | tacgccggacgcatcgtggccggcatcaccggcgccacaggtgcggttgctggcgcctatatcgccgacatcaccgatggggaagatcgggctcgccacttcgggctcatgagcgcttgtttcggcgtgggtatggtggcaggccccgtggccgggggactgttgggcgccatctcc | TACGCGGGTCGTATCGTTGCGGGTATCACCGGTGCGACCGGTGCGGTTGCGGGTGCGTACATCGCGGACATCACCGACGGTGAAGACCGTGCGCGTCACTTCGGTCTGATGTCTGCGTGCTTCGGTGTTGGTATGGTTGCGGGTCCGGTTGCGGGTGGTCTGCTGGGTGCGATCTCT | TATGCCGGAAGGATAGTCGCCGGAATAACAGGAGCCACAGGAGCCGTCGCCGGAGCCTATATAGCCGATATAACAGATGGAGAGGATAGGGCCAGGCATTTTGGACTAATGAGTGCCTGTTTTGGAGTCGGAATGGTCGCCGGACCCGTCGCCGGAGGACTACTAGGAGCCATAAGT |
| 4 | 81 | ccattccttgcggcggcggtgctcaacggcctcaacctactactgggctgcttcctaatgcaggagtcgcataagggagag | CCGTTCCTGGCGGCGGCGGTTCTGAACGGTCTGAACCTGCTGCTGGGTTGCTTCCTGATGCAGGAATCTCACAAAGGTGAA | CCCTTTCTAGCCGCCGCCGTCCTAAATGGACTAAATCTACTACTAGGATGTTTTCTAATGCAAGAGAGTCATAAGGGAGAG |
Note: All above segments have been optimized and deoptimized using the online Optimizer program.
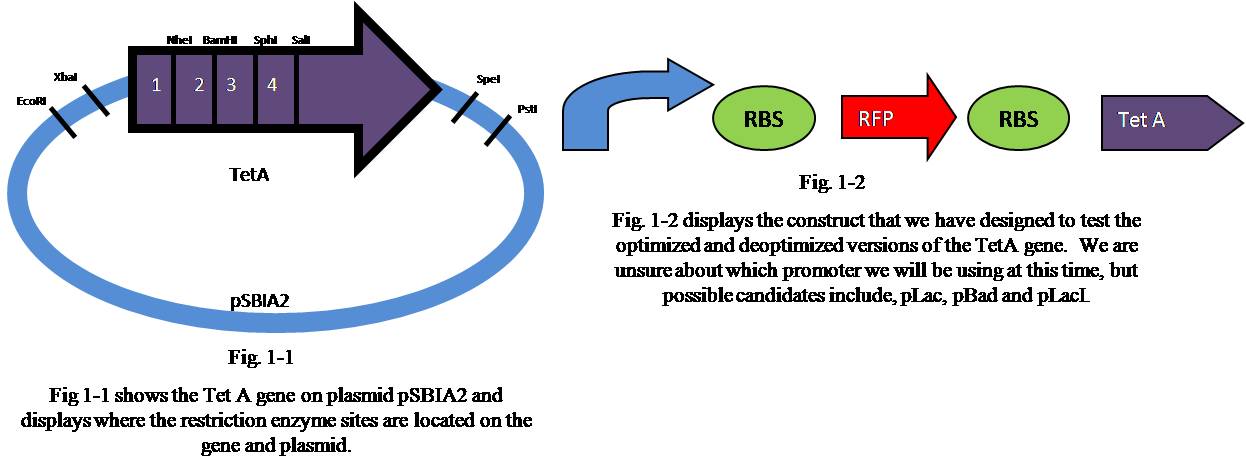
To create the optimized and deoptimized segments of the Tet A gene we used the sequence that the Optimizer program gave us and put it into the Lancelator a program created by Lance Harden as part of the 2006 iGEM team. After getting the recommended Oligo sequences from the Lancelator, we added necessary "sticky ends" that were compatible with the naturally occurring restriction enzyme sites that we discovered (see Restriction Enzymes below). At this time we have ordered Oligo parts for segments 1 and 2 of the Tet A gene. The Oligos that were ordered can be seen below.
Segment 1 Oligos
Optimized
top 1 5' AATTCGCGGCCGCTTCTAGATGAAATCTAACAACGCGCTGATCGTTATCCTGGGTACCG 3’
top 2 5' TTACCCTGGACGCGGTTGGTATCGGTCTGGTTATGCCGGTTCTGCCGGGTCTGCTGCGTGAC 3’
top 3 5' ATCGTTCACTCTGACTCTATCGCGTCTCACTACGGTGTTCTG 3’
bottom 2 5' CATAACCAGACCGATACCAACCGCGTCCAGGGTAACGGTAC CCAGGATAACGATCAGCGCGTTGTTAGATTTCATCTAGAAGCGGCCGCG 3’
bottom 1 5' CTAGCAGAACACCGTAGTGAGACGCGATAGAGTCAGAGTGAACGATGTCACGCAGCAGACCCGGCAGAACCGG 3’
Deoptimized
top 1 5' AATTCGCGGCCGCTTCTAGATGAAGAGTAATAATGCCCTAATAGTCATACTAGGAACAG 3’
top 2 5' TCACACTAGATGCCGTCGGAATAGGACTCGTCATGCCCGTCCTACCCGGACTACTAAGGGA 3’
top 3 5' TATAGTCCATAGTGATAGTATAGCCAGTCATTATGGAGTCCTG 3’
bottom 2 5' GAGTCCTATTCCGACGGCATCTAGTGTGACTGTTCCTAGTATG ACTATTAGGGCATTATTACTCTTCATCTAGAAGCGGCCGCG 3’
bottom 1 5' CTAGCAGGACTCCATAATGACTGGCTATACTATCACTATGGACTATATCCCTTAGTAGTCCGGGTAGGACGGGCATGAC 3’
Segment 2 Oligos
Optimized
top 1 5' CTAGCCTGTACGCGCTGATGCAGTTCCTGTGCGCGCCGGTTCTGGGTGCGCTGTCTGAC 3’
top 2 5' CGTTTCGGTCGTCGTCCGGTTCTGCTGGCGTCTCTGCTGGGTGCGACCATCGACTAC 3’
top 3 5' GCGATCATGGCGACCACCCCGGTTCTGTG 3’
bottom 1 5' GCGCACAGGAACTGCATCAGCGCGTACAGG 3’
bottom 2 5' CCAGCAGAACCGGACGACGACCGAAACGGTCAGACAGCGCACCCAGAACCGGC 3’
bottom 3 5' GATCCACAGAACCGGGGTGGTCGCCATGATCGCGTAGTCGATGGTCGCACCCAGCAGAGACG 3’
Deoptimized
top 1 5' CTAGCGCTATATGCCCTAATGCAATTTCTATGTGCCCCCGTCCTAGGAGCCCTAAGTG 3'
top 2 5' ATAGGTTTGGAAGGAGGCCCGTCCTACTAGCCAGTCTACTAGGAGCCACAATAGAT 3'
top 3 5' TATGCCATAATGGCCACAACACCCGTCCTATG 3'
bottom 1 5' GGCACATAGAAATTGCATTAGGGCATATAGCG 3'
bottom 2 5' TAGGACGGGCCTCCTTCCAAACCTATCACTTAGGGCTCCTAGGACGGG 3'
bottom 3 5' GATCCATAGGACGGGTGTTGTGGCCATTATGGCATAATCTATTGTGGCTCCTAGTAGACTGGCTAG 3'
Restriction Enzymes
To insert the annealed Oligo segments that were created we had to digest the Tet A gene using naturally occurring enzyme restriction sites that did not exist in the rest of the gene or in the plasmid (pSBIA2).
| Segment | First Restriction Enzyme | Second Restriction Enzyme |
|---|---|---|
| 1 | EcoRI | NheI |
| 2 | NheI | BamHI |
| 3 | BamHI | SphI |
| 4 | SphI | SalI |
Parts Used (to be used)
| Part | Registry Link | Construct Including Part |
|---|---|---|
| Tet A (tetracycline resistance gene) J31007 | Tet A (forward) | promoter-RBS-RFP-RBS-TetA on pSBIA2 |
| pLac-RBS-RFP I715039 | pLac-RBS-RFP | Possibly: pLac-RBS-RFP-RBS-TetA on pSBIA2 |
| pLac-RBS-RFP-RBS-TetA-TT on pSBIAK3 S04435 | pLac-RBS-RFP-RBS-TetA-TT | Possibly: pLac-RBS-RFP-RBS-TetA-TT on pSBIA2 |
| Part Name and Registry Number | Registry Link | Construct |
| Part Name and Registry Number | Registry Link | Construct |
Promoters
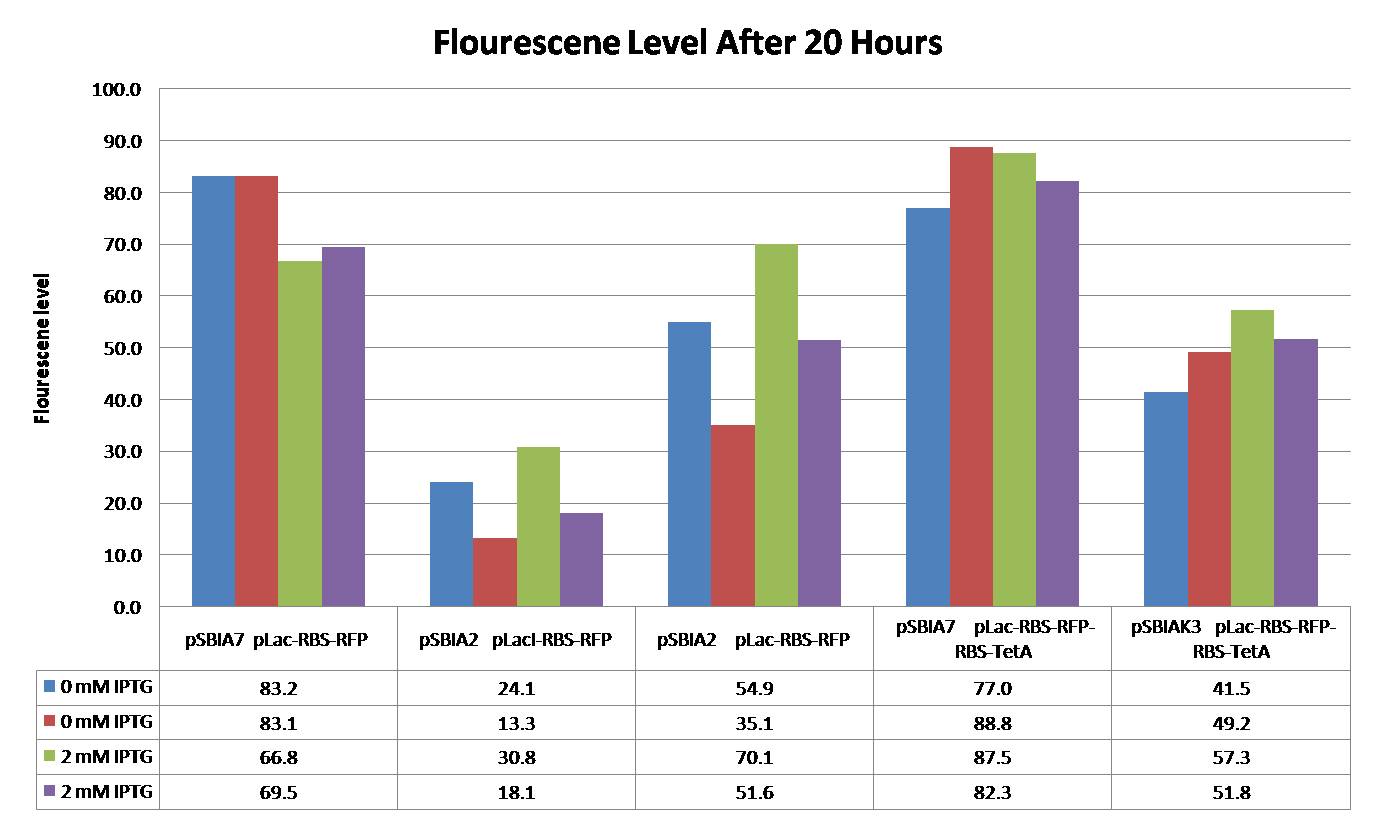
This chart shows flourescene levels of different promoter constructs with varying levels of IPTG added (error bars with standard error can be seen on the graph). Note: This experiment is still in progress. More data will be posted when available.
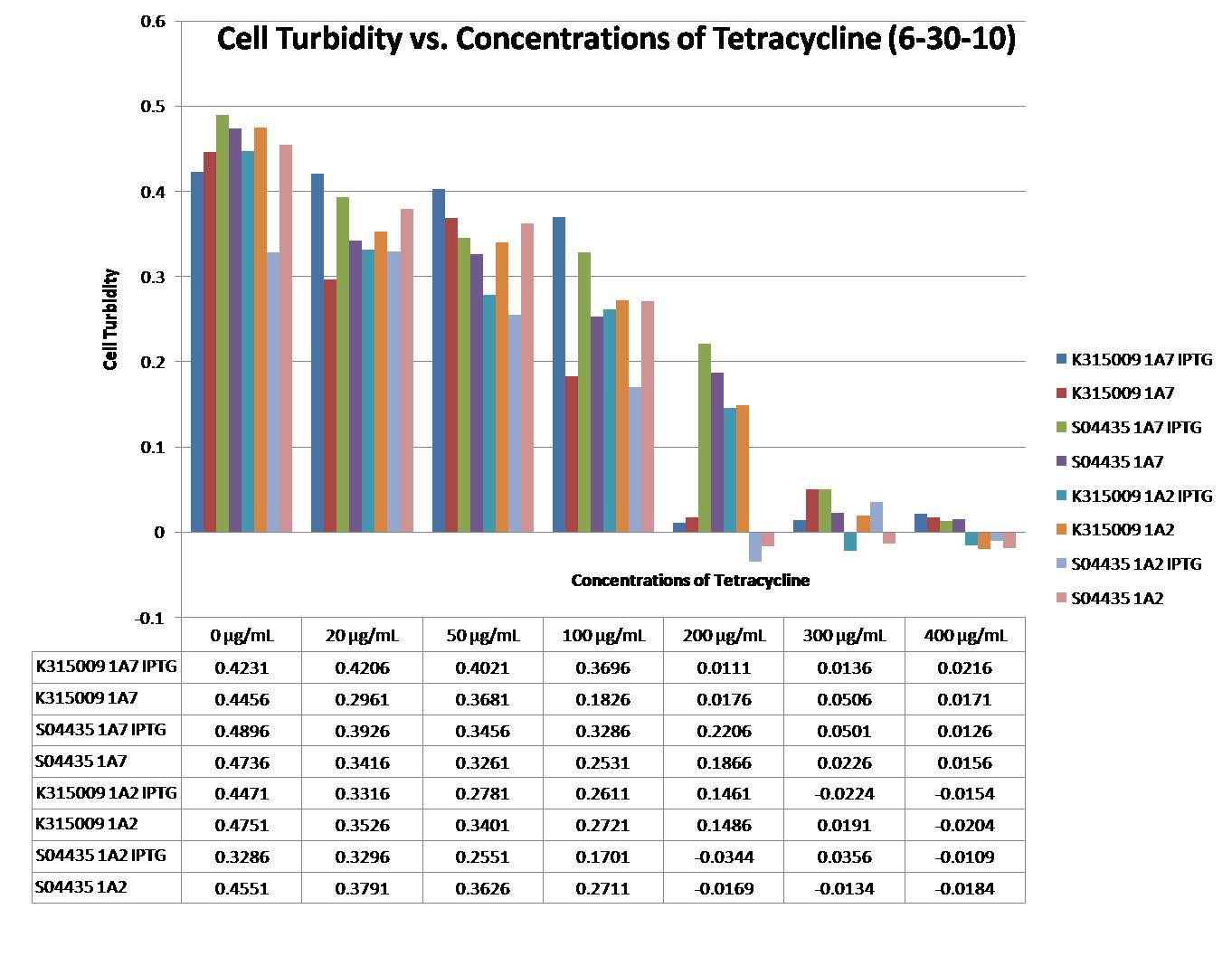
Tet-trations
Making and Testing lox_ Sites
Green Part has been cloned and sequence verified; Red Part is under construction
| Final Construct | Current Status | Registry Number and Link | Primary Team Member |
|---|---|---|---|
| lox5171 Forward in pSB1A2 | I got colonies on my second attempt. Once I isolate the plasmid, it will be sent off for sequencing. | pSB1A2 lox5171 does not exist in the registry. We ordered oligos and constructed the site in the lab. |
Nitya |
| lox5171 Reverse in pSB1A2 | This plasmid will also be sent off for sequencing soon. | pSB1A2 lox5171 does not exist in the registry. We ordered oligos and constructed the site in the lab. |
Nitya |
| loxm2 Forward in pSB1A2 | I ligated loxm2 F with the plasmid and have prepared the DNA for sequencing to confirm what is there. | [1] loxm2 does not exist in the registry. We ordered oligos and constructed the site in the lab. |
Tom |
| loxm2 Reverse in pSB1A2 | I ligated loxm2 F with the plasmid and have prepared the DNA for sequencing to confirm what is there. | [2] loxm2 does not exist in the registry. We ordered oligos and constructed the site in the lab. |
Tom |
| loxBri forward in pSB1A2 | 4 colonies grew with the correct sized insert from colony PCR verification and will be sent off for sequencing. | pSB1A2 loxBri does not exist in the registry. We ordered this oligos with 3 mutations and transformed in lab. |
Bri |
| loxBri reverse in pSB1A2 | I had 3 successful colonies from this oligos ligation that were also confirmed by colony PCR that will be sent off for sequencing. | pSB1A2 loxBri does not exist in the registry. This oligos was also transformed in the lab. |
Bri |
| loxP reverse in pSB1A2 | I had 5 successful colonies from this oligos ligation that were also confirmed by colony PCR. They will be sent off for sequencing. | loxP reverse loxP reverse exists in the registry. We ordered this oligos and ligated it into pSB1A2 in the lab. |
Bri |
| loxN Forward in pSB1A2 | I had 5 successful colonies from this oligos ligation that were also confirmed by colony PCR. They are sent off for sequencing and four of them were confirmed to be correct. | [3] loxN forward does not exist in the registry. We ordered the oligos and built it in the lab. |
Anvi |
| loxN Reverse in pSB1A2 | I had 3 successful colonies from this oligos ligation that were also confirmed by colony PCR. They are sent off for sequencing and two of them were confirmed to be correct. | [4] loxN reverse does not exist in the registry. We ordered the oligos and built it in the lab. |
Anvi |
| lox2272 Reverse in pSB1A2 | I had 1 successful colony from this oligos ligation that were also confirmed by sequencing and its cells have been plate on LB Amp plates and frozen into freezer stocks. | [5] lox2272 reverse does not exist in the registry. We ordered the oligos and built it in the lab. |
Jamela |
| lox2272 Forward in pSB1A2 | I had 3 successful colony from this oligos ligation that were also confirmed by sequencing and its cells have been plate on LB Amp plates and frozen into freezer stocks. | [6] lox2272 foward does not exist in the registry. We ordered the oligos and built it in the lab. |
Jamela
|
Testing Cre Activity
Green Part has been cloned and sequence verified; Red Part is under construction
| Final Construct | Current Status | Registry Number and Link | Primary Team Member |
|---|---|---|---|
| I718008 (pBAD+RBS+cre) in pSB4K5 | At this point, I have isolated both the insert and the plasmid, but my two attempts at ligation have not produced any colonies. | I718008 pSB4K5 |
Nitya |
| I718008 (pBAD+RBS+cre) in pSB3K3 | At this point, I have ligated and transformed the cells but I am having trouble growing it in the LB + kan media. | I718008[7] describe intermediate |
Anvi |
| Insert Info Here | what is built so far? | change to correct link describe intermediate |
your name here |
| Insert Info Here | what is built so far? | change to correct link describe intermediate |
your name here |
| Insert Info Here | what is built so far? | change to correct link describe intermediate |
your name here |
| Insert Info Here | what is built so far? | change to correct link describe intermediate |
your name here |
| Insert Info Here | what is built so far? | change to correct link describe intermediate |
your name here |
| Insert Info Here | what is built so far? | change to correct link describe intermediate |
your name here |
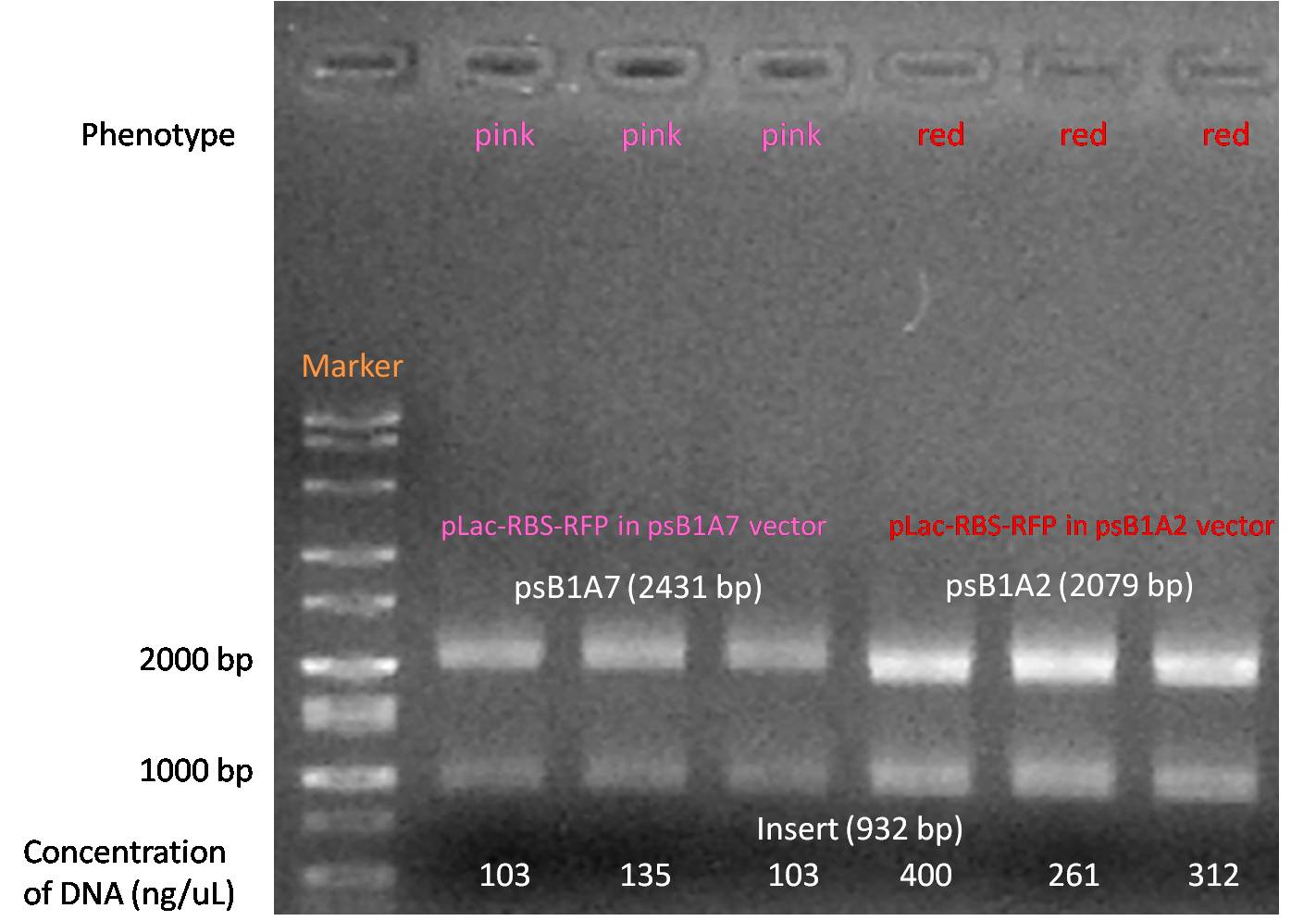
pSBIA7 & pSBIA2
Data showing the varying levels of expression between pSBIA7 and pSBIA2