Sarah's Assignment
I'm interested in the DNA repair mechanisms our species might have. Since or species is in such exposed conditions, how does it evade the mutational effects of UV light?
"UV radiation produces many types of photochemical alterations in DNA, ribonucleic acid (RNA), and protein, as well as to structures such as membranes. DNA, however, is the major target for the deleterious effects of UV radiation because it is the largest molecule in the cell, it is present in the fewest copies, it carries the genetic information for a cell, and it absorbs UV radiation very efficiently. In contrast, RNA and proteins are present in multiple copies, so that it is much more difficult to inactivate a significant number of these molecules in a cell by UV irradiation. "[1]
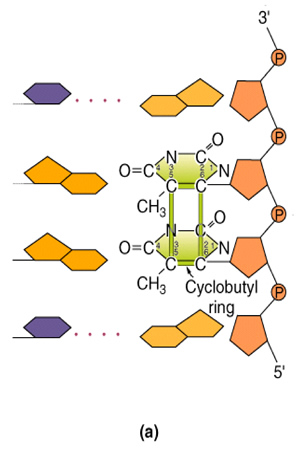
Commonly found UV-mediated mutations are cyclobutane dimers (pyrimidine dimers) and 6-4 photodimers (or 6,4 photoproducts).
Figure 1: Example of a cyclobutane dimer.
Cyclobutane dimers consist of either T to T (most common), C to T or (less common) C to C. UV light creates covalent bonds between adjacent thymidines or cytosines, which can inhibit transcription or replication of DNA. Cytosines that are part of a dimer are also more likely to be deaminated and changed to uracil. This can cause errors in either transcription or replication [2] [3].
Deoxyribodipyrimidine photolyases are partially responsible for correcting cyclobutane dimers by breaking the covalent bonds formed by UV light exposure. The reaction they perform can be characterized by: cyclobutadipyrimidine (in DNA) = 2 pyrimidine residues (in DNA) [4]. Interestingly, these photolysases are not found in placental mammals.
From JGI
According to JGI, our species has a deoxyribodipyrimidine photolyase (644033060), a deoxyribodipyrimidine photolyase-related protein (644030931) and a DNA repair photolyase (644032076). In other halophiles, it has been determined that photolyases are light dependent and DNA repair more efficient in the presence of light [5]. Since mutations from UV light are more likely to occur during the day, it stands to reason that the repair mechanism would be light-dependent and function only when necessary.
According to JGI our species also has the following genes associated with DNA repair:
5 DNA mismatch repair enzymes
2 DNA oxidative protectant proteins
Endonucleases V and IV
1 DNA repair exonuclease
3 glycosylases
I considered focusing on all of these genes but decided a more specific focus was necessary for the most concise and effective results. I chose to pursue DDP because it is a one-enzyme pathway. I thought a simpler protein would allow me to more easily make comparisons to other genomes. As a secondary step, I wanted to look at the glycosylases as well since it is there job to recognize certain mutations in the genome and their increased performance may assist in our species' ability to combat the negative effects of UV radiation.
BLASTn
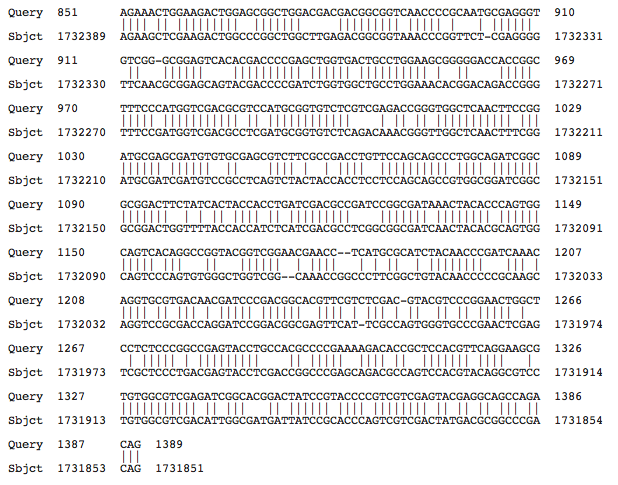
BLASTing the first dexyribidipyrimidine photolyase brought up a complete match with H. mukohaetaei and loose identification with H. marismortui as shown below:
While the similarities are obvious and the BLAST supports that this is, in fact, a deoxyribodipyrimidine photolyase, there are many differences between the two halophiles.
BLASTs with this other two potential deoxyribodipyrimidine photolyases were not as promising:
Deoxyribodipyrimidine photolyase-related protein:
Only one other whole genome showed significant similarity.
DNA repair photolyase:
Our gene was only matched to its own genome.
BLASTp
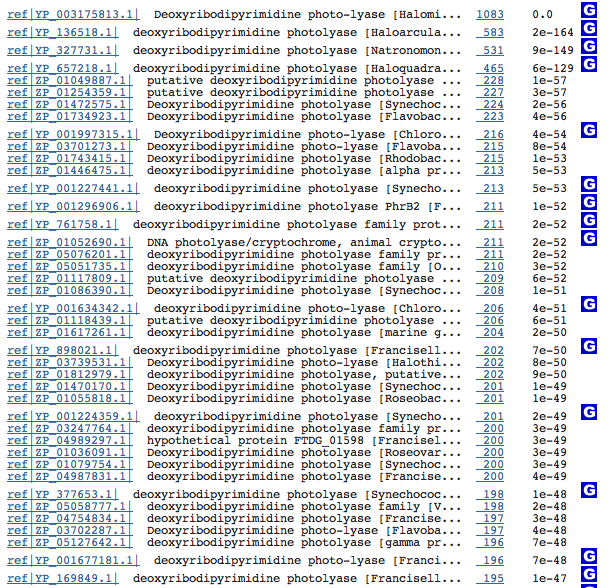
Blasting the amino acid sequence for each of the three possible photolyases brought more promising results.
DDP:
DDP-related protein:
DNA repair photolyase:
Strangely, when the amino acid sequences from the DDPs found in our species' genome were BLASTed against one another, very little similarity was found.
Most of the organisms that showed significant similarity with our organism's DDP were also halophiles found in salt flats around the world. This suggests that maybe the changes which make our organism's DNA-repair process so efficient have developed independently in many halophiles, while the differences show how different environmental pressures have tweaked the enzymes to fit precise needs.
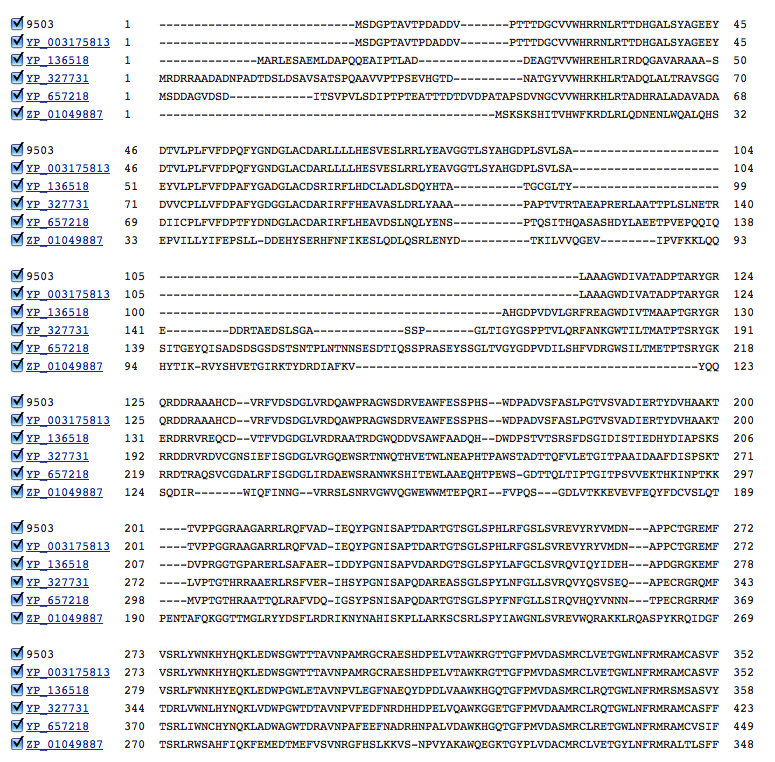
I then performed a COBALT multiple alignment of the amino acid sequences of the first 5 most significant alignments with the following results:
It's clear that there are some highly-conserved areas across all of the DDPs in these halophiles. I would like to look at these conserved regions in greater detail and compare them to non-halophiles to determine how these areas of the protein differ and how that might affect their effectiveness.
I tried to figure out what structures these form in the folded protein but the process is proving difficult.
To edit: Found several papers that discuss halophiles and their mutational rates in response to UV light or radiation [6].
From RAST
According to RAST, our species has 8 enzymes involved in base excision DNA repair. 1 glycosylase, 4 endonucleases, 1 exonuclease and 2 ligases [7]. JGI suggests Base Excision Repair as the only method our organism has for DNA repair and makes no mention of DDP.
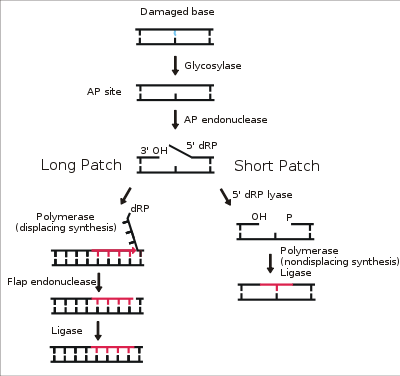
Figure2. A simplified example of Base Excision Repair (BER).
in BER, glycosylases recognize errors in DNA base-pairing. Endonucleases cleave the 3' end of the error and endonucleases cleave the 5' end. DNA polymerase starts re-building the correct complementary strand and flips the excised bases away from the double helix so that the endonuclease can recognize and cleave it. In single base-pair excisions, lyase removes the entire incorrect base and DNA polymerase fills in the gap. In both cases, ligases create the covalent bond between the old DNA strand and the last nucleotide placed by DNA polymerase.[8]
One of the endonucleases is a flap structure-specific endonuclease. These enzymes are used during the excision of longer sequences of DNA suggesting that our species is capable of more than single-nucleotide excision repair.
Comparison of RAST and JGI
I find it very interesting that, so far, I have not found any similar genes between RAST and JGI. I would like to probe further into this issue and try to find common endonucleases and photolyases in both annotations. I am open to suggestions as to which specific endonucleases (or other genes) I should compare.
While both JGI and RAST recognize endonucleases in the genome, RAST recognizes only Endonuclease III and a flap structure-specific nuclease. whereas JGI recognizes endonucleases IV and V.
I BLASTed the first photolyase gene against the entire H. mukohaetaei genome in the RAST portal and found alignment, but RAST did not identify this section of the genome as a gene.
What this means
Why do we care about our species' ability to withstand the mutations caused by UV radiation?
Our species presents us with a more efficient version of an old system. Most organisms on the planet use photolyases to correct UV-radiation DNA damage but the halophiles have adapted to their decidedly harsh environment and developed a better compilation of potentially more efficient enzymes to combat this problem.
As we have talked about in class, laboratory conditions are decidedly different from conditions in the "real world". As scientists work to create new organisms that could potentially help in real world situations, they must take into account real world conditions. Most laboratories do not have high levels of UV radiation, unlike the open ocean or a waste dump, yet these are the places that engineered organisms are being sent. If they are unable to survive in the environment they were created to help, then what good can they do? These organisms are also created to very exact specifications. Mutations that do not kill them might render them incapable of performing the function they were meant to or might even alter that function (potentially in a harmful way). WIth knowledge of our organism's (and other halophile's) more efficient UV-radiation-induced DNA damage repair, perhaps genomicists will be able to assist in the development of better bio-tools.
Questions
Which sections of the DDP protein are highly conserved among halophiles?
What section of the protein does the conserved sequence reside in?
What differences/similarities are there between halophilic and non-halophilic photolyases?
How does halophilic (specifically our organism's) DNA repair differ from the repair processes of other organisms?
Is it relevant that our organism has 3 photolyases?
What does it say about RAST and JGI that they annotated completely different genes?