WEEK FOUR (February 6 - 10)
We have been given the DNA encoding 18 genes in 10 different clones and we need to get the DNA into cells, verify the inserts, freeze down glycerol stocks, enter them into GCAT-alog, and send some cells to our colleagues at MWSU.
- Everyone should register for a GCAT-alog account (v2.5.4 or higher).
- The plan this week is to do all wet lab stuff. Dr. Heyer will be out of town and we need to get the magnetasome DNA into E. coli cells (strain JM109). I know none of you can come to all these meetings, but we will be doing each step as outlined over the week. Come to the ones you can attend. Each person should be able to do at least 3 of these. You can choose which three.
Friday February 3 9:30 am
transform JM109 & plate on appropriate media. (Confirm with UW team)
take about 45 minutes
Saturday February 4 I need one volunteer
take out of incubator put in fridge
take about 15 minutes and must be done before 9 am
Sunday February 5 mid-afternoon: 3 pm
pick colonies from each plate and put into 2 mL appropriate media
take about 30 minutes
Monday February 6 4:30 pm
Do minipreps but SAVE THE CELLS on plates
takes about 1 hour
Tuesday February 7 4:30 pm
set up digestions, run 30 min to 1 hr and put in fridge
Determine insert sizes from Registry
Wednesday February 8 4:30 pm
determine the percent agarose needed for the inserts
pour and run gel(s) then photograph gel(s)
verify insert sizes (get this from Registry)
molecular weight marker
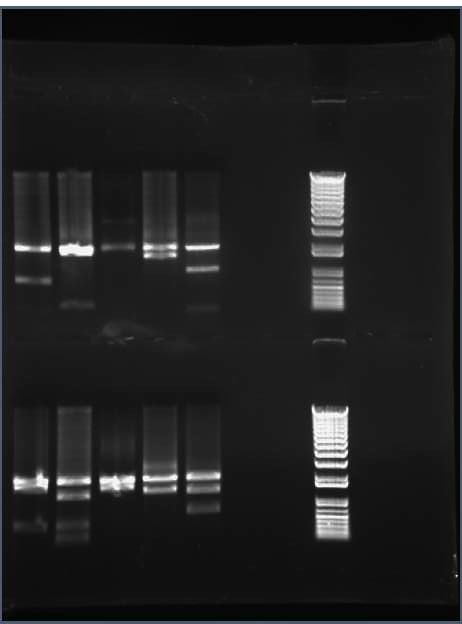
mamE = 2253 bp, insert looks correct based on gel (Ben)
mamO = 1914 bp or 1975 bp insert looks correct based on gel (Erich)
mamQRB = 2029 bp or 2108 bp, insert looks correct based on gel (Dancho)
mamPA = 1584 bp, insert looks correct based on gel (Ellen)
mamV = 1168 bp, insert looks correct based on gel (Malcolm)
mamSTU = 2030 bp or 2160 bp, insert looks correct based on gel (Kirsten)
mamMN = 2323 bp or 2367 bp, insert looks correct based on gel (Nishita)
mamJ = 1521 bp or 1626 bp (Becca)
mamKL= 1336 or 1439 bp insert looks correct base on gel (Alex)
mamHI = 1600 bp or 1626 (Duke) -- insert looks incorrect
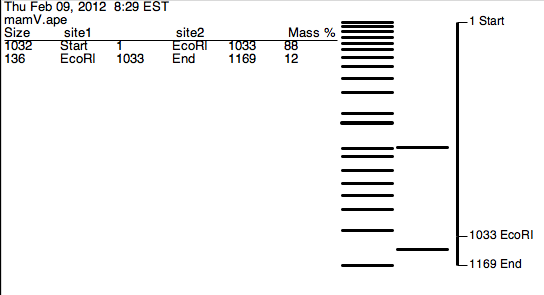
ApE digestion of mamV using BamHI (0 sites) and EcoRI (1 site) produces two fragments of 1032 bp and 136 bp.
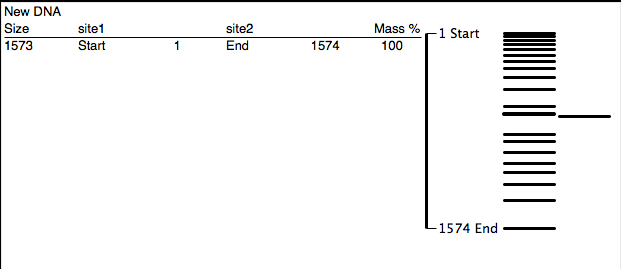
ApE digestion of mamPA using BamHI (0 sites) and EcoRI (0 sites) produces one fragment of 1573 bp.
Thursday February 9 11 am
grow overnight cultures of clones with correct inserts sizes
pick from plate and put into 2 mL of appropriate media
streak out on new plate for shipping to MWSU.
Friday February 10 4 pm
take cells out of incubator
freeze down cells and enter into GCAT-alog.
Primers used for fragment extraction:
mamHI_fwd GGTACCTTCGTATGAACCCTGTG
mamHI_rev CGTCTTCTACGTCACCATTGAAC
mamE_fwd CCGCTTCAGACCCTGACA
mamE_rev CGATCTCGCCGGTTATTC
mamJ_fwd CAGATTTTGCTGAAGGTCAACC
mamJ_rev CGTTCGCGTGCTAAATGAC
mamKL_fwd CTGGCAGCCGTCAATTG
mamKL_rev CCTCATCCTTACTCACTCCAAAGC
mamMN_fwd ATCCCTTCGCTTGGGTTG
mamMN_rev AATCATGGCTGAGTTCCAAGC
mamO_fwd GAGATGACGACAGGAATCCG
mamO_rev CCAATCCCAGCATCATGATC
mamPA_fwd TGCTGACCTCGGTGTGATG
mamPA_rev GAAGGAAACGCCCCACATAC
mamQRB_fwd CTTGCCGCATTTCAAGAAG
mamQRB_rev GGCTCAACATACGCTCTGG
mamSTU_fwd CGCATCCAGGAGGAAATC
mamSTU_rev AACCGCACCACCTTGC
mamV_fwd GCTGGTGCCCAAATAATCG
mamV_rev CAATCGCCAACAGCGTAG
media:MamAB_copy.txt ApE file for the mamAB operon]] with the primers, genes, native promoter, and terminator sequences annotated. Right click and save as, then open with ApE (A plasmid Editor).
media:pGA1C3_pLac-GFP.txt pGA1C3 plasmid map (with pLac GFP insert). Right click and save as, then open with ApE (A plasmid Editor).
mamV = 1002 bp or 1168 bp, depending on how we verify the insert. (Malcolm)
mamO = 1914 bp or 1975 bp (Erich)
mamPA = 1493 bp or 1584 bp (Ellen)
mamSTU = 2030 bp or 2160 bp (Kirsten)
mamQRB = 2029 bp or 2108 bp (Dancho)
mamE = 2172 bp or 2253 bp (Ben)
mamHI = 1600 bp or 1626 (Duke)
mamKL= 1336 or 1439 bp (Alex)
mamMN = 2323 bp or 2367 bp (Nishita)
mamJ = 1521 bp or 1626 bp (Becca)