Difference between revisions of "Riboswitches"
(→From Concept to Wet Lab) |
(→From Concept to Wet Lab) |
||
| Line 25: | Line 25: | ||
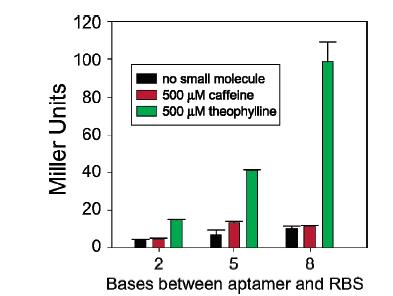
'''Figure 16:''' Cells exposed to theophylline (green) showed greater beta-galactosidase activity than cells exposed to caffeine (red) or cells exposed to nothing (black) no matter the distance of the riboswitch from the RBS, but the greatest increase was experienced by cells with the riboswitch 8 base pairs (the farthest tested distance) from the RBS exposed to theophylline. | '''Figure 16:''' Cells exposed to theophylline (green) showed greater beta-galactosidase activity than cells exposed to caffeine (red) or cells exposed to nothing (black) no matter the distance of the riboswitch from the RBS, but the greatest increase was experienced by cells with the riboswitch 8 base pairs (the farthest tested distance) from the RBS exposed to theophylline. | ||
| + | |||
| + | [Post-transcriptional Regulation Technologies - Erin Zwack|Return to Post-transcriptional Regulation Technologies] | ||
Revision as of 23:59, 5 December 2007
Riboswitches are small sequences in mRNA that bind small molecules to regulate translation and occasionally transcription. Riboswitches occur naturally in both eukaryotes and prokaryotes. Desai and Gallivan hoped to find new synthetic riboswitches (riboswitches with new ligand specificities) by creating libraries of mutant riboswitches and using genetic selection to pick the functional ones of interest. Desai and Gallivan also employed riboswitches to screen for the presence of specific small molecules. In theory riboswitches are perfect because the number of aptamers already in existence and our capbaility to engineer new aptamers through rational design.
Design
Reviews of previous research showed that the theophylline aptamer worked in riboswitches in wheat germ, a eukaryote, and Bacillus subtilis, a gram positive bacterium. Desai and Gallivan decided to translate the technology to gram negative bacteria. To do this they cloned the theophylline aptamer five base pairs upstream of the RBS for lacZ. The gene is controlled by a weak promoter and a weak RBS allowing for sensitivity to changes in translation because of the presence of theophylline. The construct was then transformed into E. coli. When theophylline is added, translation should be turned on again.
From Concept to Wet Lab
The aptamer for theophylline was inserted in front of the gene for beta-galactosidase gene to create an mRNA with a riboswitch. Beta-dalactosidase activity is dependent on the amount of the enzyme beta-galactosidase; therefore, measurements of beta-galactosidase indicate whether translation of the mRNA is taking place. Testing and comparing beta-galactosidase activity for cells with the riboswitch in the presence of theophylline, caffeine, and no ligand as well as testing and comparing beta-galactosidase activity for cells without the riboswitch under the same conditions demonstrated that the riboswitch does control gene expression in response to theophylline (figure 14). Significant increases in beta-galactosidase activity in cells with the riboswitch were only found when theophylline was added. Because beta-galactosidase activity in the presence of theophylline, caffeine, or no ligand for cells without a riboswitch did not significantly vary, the increase in beta-galactosidase activity in the presence of theophylline for cells with a riboswitch most likely resulted from the theophylline binding to the riboswitch and allowing translation.
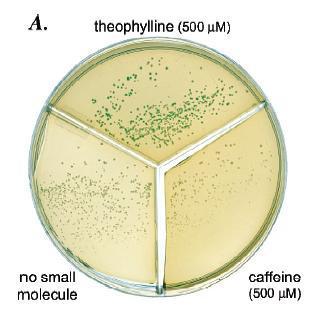
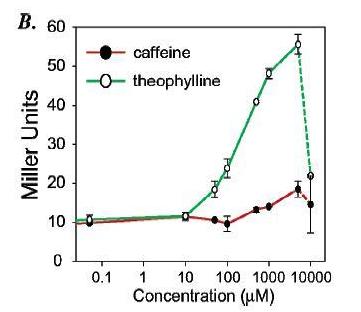
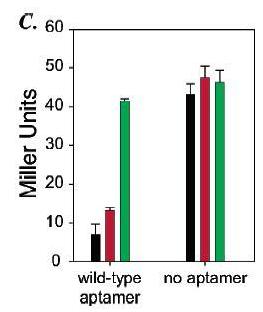
Figure 14: a) On the plate are cells with the riboswitch that were grown in three different conditions: theophylline present, caffeine present, and no ligand present. Cells that were grown in the presence of theophylline have a distinct blue/green color, which indicates beta-galactosidase activity, compared to the cells grown with caffeine or without ligand. The lack of color in the cells grown with caffeine or without ligand indicates that the riboswitch most likely prevents translation of the mRNA encoding beta-galactosidase. The blue color of the cells grown with theophylline indicates that theophylline probably is binding to the riboswitch and allowing for translation. b) The green line represents cells that have had theophylline added to them while the red line represents cells that had caffeine added. Beta-galactosidase activity's increasing, as measured by Miller Units, significantly only in the presence of high enough concentrations of theophylline but not caffeine suggests that the riboswitch is highly specific for its ligand. c) When no riboswitch existed in the beta-galactosidase transcript, beta-galactosidase activity was not significantly different for cells grown in the presence of theophylline, caffeine, or no ligand. When a riboswitch existed in the beta-galactosidase transcript, cells grown in the presence of theophylline exhibited significantly greater beta-galactosidase activity than cells grown in the presence of caffeine or no ligand. Beta-galactosidase activity for cells grown in the presence of caffeine did not vary significantly from activity for cells grown without any ligand present.
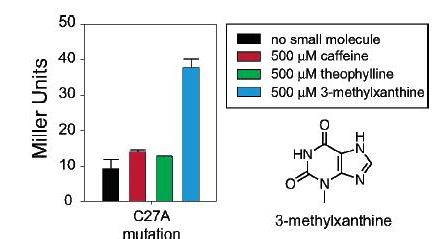
To further support that the theophylline was interacting with the aptamer and not just increasing protein translation through another route, a single point mutation that in vitro decreases affinity for theophylline and increases affinity for 3-methylxanthine was introduced to the aptamer. When using this mutant riboswitch, the beta-galactosidase activity, in the presence of theophylline now was almost the same as beta-galactosidase activity without any small molecule while beta-galactosidase in the presence of 3-methylxanthine was significantly higher than base-line. These results suggest that the change in translation as indicated by the increase of beta-galactosidase is controlled by the riboswitch and not some other mechanism.
Figure 15: Beta-galactosidase activity was low and basically the same for cells with the mutant riboswitch that had no molecule (black), caffeine (red), or theophylline (green) added. Cells with the mutant riboswitch showed significant increase in beta-galactosidase activity when 3-methylxanthine (blue) was added.
In synthetic biology, parts are often tweaked until the efficiency, detectability, and other properties are enhanced to work as a part in a device. Gallavin's lab worked on optimization of the riboswitch by changing the position of the aptamer in relation to the RBS. The riboswitch showed a greater increase in beta-galactosidase activity when the aptamer was 8 base pairs upstream of the RBS than when the aptamer was either 2 or 5 base pairs upstream. This result could be because of the surrounding bases being purines in this particular transcript or the actual distance.
Figure 16: Cells exposed to theophylline (green) showed greater beta-galactosidase activity than cells exposed to caffeine (red) or cells exposed to nothing (black) no matter the distance of the riboswitch from the RBS, but the greatest increase was experienced by cells with the riboswitch 8 base pairs (the farthest tested distance) from the RBS exposed to theophylline.
[Post-transcriptional Regulation Technologies - Erin Zwack|Return to Post-transcriptional Regulation Technologies]