Difference between revisions of "Antiswitches"
(→Design) |
(→Design) |
||
| Line 26: | Line 26: | ||
The aptamer stem is a short sequence that complements a portion of the sequestering sequence of the antisense stem. In an off-switch, the aptamer stem swings towards the antisense stem and displaces the portion that complements the targeted transcript when the liand binds to the aptamer. In an on-switch, the aptamer swings away from the antisense stem when the ligand bids to the aptamer; thus, the antisense stem can duplex and is no longer free to bind to the transcript. | The aptamer stem is a short sequence that complements a portion of the sequestering sequence of the antisense stem. In an off-switch, the aptamer stem swings towards the antisense stem and displaces the portion that complements the targeted transcript when the liand binds to the aptamer. In an on-switch, the aptamer swings away from the antisense stem when the ligand bids to the aptamer; thus, the antisense stem can duplex and is no longer free to bind to the transcript. | ||
| − | [[Image: | + | [[Image:ANTI.JPG]] |
==From Concept to Wet Lab== | ==From Concept to Wet Lab== | ||
Revision as of 04:31, 5 December 2007
Antiswitches, trans-RNA molecules that regulate translation of mRNA based on ligands, were first developed by Smolke and Bayer (2005) in Saccharomyces cerevisiae. Two types of antiswitches were engineered: on-switches and off-switches. On-switches turn on gene expression in the presence of the ligand while off-switches turn off gene expression in the presence of ligand. Control by the ligand allows researchers to regulate pathways’ protein production with less leakiness,low level transcription even when “off”, than using a specific promoter.
Design
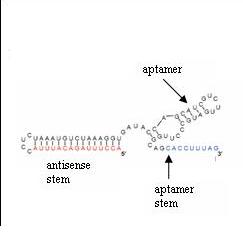
Antiswitches are made of an aptamer and two stems: the aptamer stem and the antisense stem (figure 3).
Figure 3: The antisense stem can bind to itself (duplex). The antisense stem also contains the complement to the RNA that a researcher desires to regulate. The aptamer stem swings either toward the antisense stem and disrupts the duplex or swings away from the antisense stem and allows the antisense stem to duplex depending on whether the aptamer is bound to its ligand.
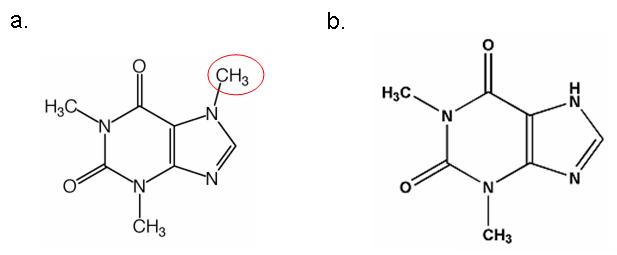
The aptamer is the sequence that binds the ligand and causes a conformational (shape) change of the antiswitch molecule. Aptamers can be highly specific for their particular molecules. The theophylline aptamer used by Smolke and Bayer can distinguish between caffeine and theophylline, which differ by a single methyl.
Figure 4. Caffeine (a) and Theophylline (b) differ by one methyl group (circled in red).
While Smolke and Bayer mainly used the aptamer for the ligand theophylline, aptamers for many other molecules are now being generated using rational design. The large number of possible aptamers provides versatility in what will control gene expression.
The antisense stem contains a sequence that complements a targeted RNA transcript and a second sequence that sequesters this complementary sequence to keep it from binding the transcript. When the antisense stem is not duplexed with itself, it prevents translation by binding to the complementary mRNA.
The aptamer stem is a short sequence that complements a portion of the sequestering sequence of the antisense stem. In an off-switch, the aptamer stem swings towards the antisense stem and displaces the portion that complements the targeted transcript when the liand binds to the aptamer. In an on-switch, the aptamer swings away from the antisense stem when the ligand bids to the aptamer; thus, the antisense stem can duplex and is no longer free to bind to the transcript.
From Concept to Wet Lab
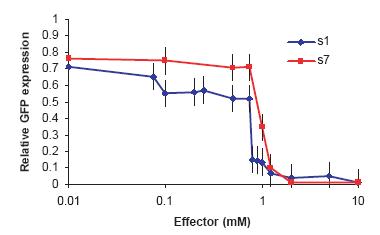
When enough ligand (~ 1 mM theophylline or tetracycline depending on the switch) was present, the inactive off-switches became active and relative GFP expression dropped to almost zero. These experimental results support the antiswitch technology being functional. As off-switches with theophylline and tetracycline binding aptamers worked, the versatility of antiswitches is supported.
Red line = tetracycline controlled switch
Blue line = theophylline controlled switch
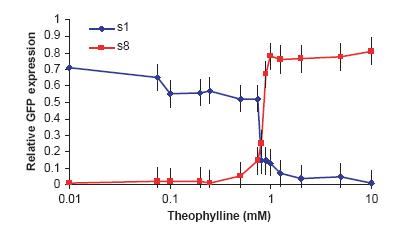
When ~ 1 mM of theophylline was added to cells containing the inactive on-switch, the cells relative expression of GFP jumped from near zero to approximately 0.9. This data show that the on-switch is also functional.
Red Line = onswitch
Blue Line = offswitch
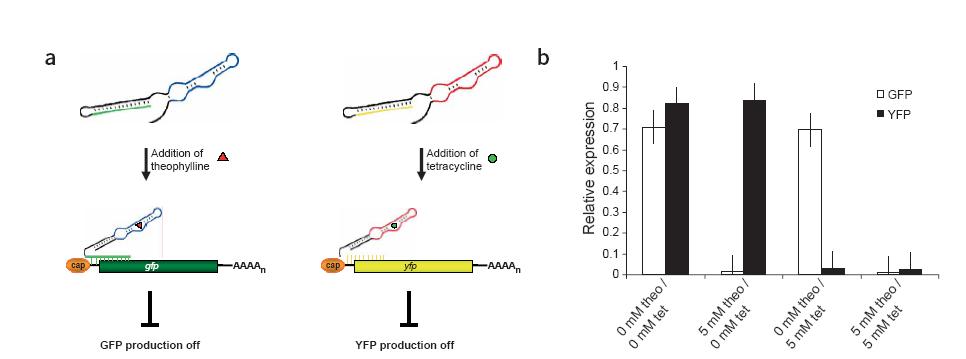
Finally, antiswitches in eukaryotes can be combined to regulate multiple genes for different conditions like the taRNA/crRNA system in prokaryotes. By simply switching the aptamer and antisense stem targeting sequence, the switch now controls a different gene by a different stimulus. Off-switch experiments using a theophylline aptamer with a GFP targetin antisense stem and a tetracycline aptamer with a YFP targeting antisense stem showed that the switches regulate the genes independently and can be used in combination to create complex regulatory mechanisms.