Difference between revisions of "Davidson Projects with Updates"
m (→Building LsrK receiver '''TESTER''' cell) |
(→Building LuxS sender '''TESTER''' cell) |
||
| Line 9: | Line 9: | ||
== Building LuxS sender '''TESTER''' cell == | == Building LuxS sender '''TESTER''' cell == | ||
| + | |||
| + | Parts to be built <br> | ||
| + | LacIpL + B0034 + LuxS | ||
| + | |||
| + | LuxS is the synthase protein for AI-2. This part is not available in the registry. The promoter and ribosome binding site were taken from the LuxR tester system. The promoter is part R0011. | ||
== Building LsrK/LsrR receiver '''TESTER''' cell == | == Building LsrK/LsrR receiver '''TESTER''' cell == | ||
Revision as of 15:05, 24 June 2008
Contents
- 1 Building LuxI sender TESTER cell
- 2 Building LuxR receiver TESTER cell
- 3 Building LuxS sender TESTER cell
- 4 Building LsrK/LsrR receiver TESTER cell
- 5 Building LasI sender TESTER cell
- 6 Building LasR receiver TESTER cell
- 7 Testing Crosstalk between Lux and Las
- 8 AND gate using pTetLac promoter
- 9 Building XOR Gate
- 10 Testing XOR Gate
- 11 Making Better pLac promoter and LacI proteins
Building LuxI sender TESTER cell
R0011 (verified) + F1610 (not right part from registry)
Going after S03608 (pLac_RBS_LuxI) from 2008 paper registry :-(
If we get this and verify, we are done with this. If not, we are in need of LuxI part.
Building LuxR receiver TESTER cell
Part K09100 has been built.
Ready to test.
Building LuxS sender TESTER cell
Parts to be built
LacIpL + B0034 + LuxS
LuxS is the synthase protein for AI-2. This part is not available in the registry. The promoter and ribosome binding site were taken from the LuxR tester system. The promoter is part R0011.
Building LsrK/LsrR receiver TESTER cell
Building LasI sender TESTER cell
Building LasR receiver TESTER cell
Ligatiion 1A S03156 + B0015
Ligation 1B R0079 + E0240
Ligation 1C B0015 + R0079
Ligation 2: Successful Ligation 1A + uccessful Ligation 1B
Ligation 3: Successful Ligation 2 + R0011
Testing Crosstalk between Lux and Las
1) Mix LuxI sender cells with LasR receiver tester cells: negative control
2) Mix LasI sender cells with LuxR receiver tester cells: negative control
3) Mix LuxI sender cells with LuxR receiver tester cells: experiment we hope will glow green
4) Mix LasI sender cells with LasR receiver tester cells: experiment we hope will glow green
5) Verify LuxR receiver tester cells don't glow solo
6) Verify LasR receiver tester cells don't glow solo
AND gate using pTetLac promoter
We have successfully built the pTetLac AND promoter: K091101.
This is sequence verified.
Now we need the various LacI proteins (I12, X86 and I12+X86 variants).
We need tetR repressor protein. We have tried to get it from the paper registry.
We need to see if this AND gate works as designed.
We need to test output to see if the two halves are balanced.
Building XOR Gate
List of auto-inducers and their catalog numbers.
Here is an idea Malcolm and Laurie developed.
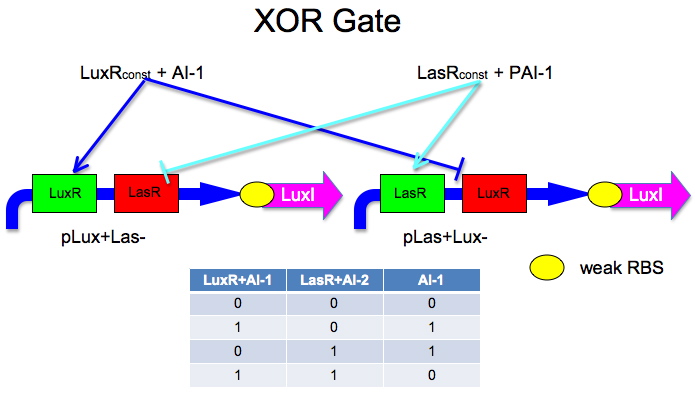
The idea is to have two mirrored halves of the system. LasR is regulated by PAI-1 {3-oxododecanoyl-HSL (3OC12HSL)} and LuxR is activated by AI-1 {3-oxohexanoyl-homoserine lactone (3OC6HSL)}. There is a potential problem in that the Lux half is more likely to get positive feedback than the Las half. This MAY not be a problem because 0/0 is leaky so we put a weak RBS to minimize leaky protein production. Also, if we add AI-2 and AI-1 is produced by leak, then the entire system shuts down. The repressor site is located between -35 and -10 of the promoter. The activator binding site is upstream of -35. This has been documented by Egland and Greenberg
Oligos_to_Build: Sequences we will need to make this XOR gate.
Testing XOR Gate
We will need to have constitutive LasR and LuxR made in these cells.
Then we will need to hit them with the two chemicals AHL types and commercial sources.
Making Better pLac promoter and LacI proteins
LacI wild-type gene sequence (ORF begins at the first amino acid)
ATGAAACCAGTAACGTTATACGATGTCGCAGAGTATGCCGGTGTCTCTTATCAGACCGTTTCCCGCGTGGTGAACCAGGCCAGCCACGTTTCTGCGAAAACGCGGGAAAAAGTGGAAGC
GGCGATGGCGGAGCTGAATTACATTCCCAACCGCGTGGCACAACAACTGGCGGGCAAACAGTCGTTGCTGATTGGCGTTGCCACCTCCAGTCTGGCCCTGCACGCGCCGTCGCAA
ATTGTCGCGGCGATTAAATCTCGCGCCGATCAACTGGGTGCCAGCGTGGTGGTGTCGATGGTAGAACGAAGCGGCGTCGAAGCCTGTAAAGCGGCGGTGCACAATCTTCTCGCGC
AACGCGTCAGTGGGCTGATCATTAACTATCCGCTGGATGACCAGGATGCCATTGCTGTGGAAGCTGCCTGCACTAATGTTCCGGCGTTATTTCTTGATGTCTCTGACCAGACACCATCA
ACAGTATTATTTTCTCCCATGAAGACGGTACGCGACTGGGCGTGGAGCATCTGGTCGCATTGGGTCACCAGCAAATCGCGCTGTTAGCGGGCCCATTAAGTTCTGTCTCGGCG
CGTCTGCGTCTGGCTGGCTGGCATAAATATCTCACTCGCAATCAAATTCAGCCGATAGCGGAACGGGAAGGCGACTGGAGTGCCATGTCCGGTTTTCAACAAACCATGCAAATGC
TGAATGAGGGCATCGTTCCCACTGCGATGCTGGTTGCCAACGATCAGATGGCGCTGGGCGCAATGCGCGCCATTACCGAGTCCGGGCTGCGCGTTGGTGCGGATATCTCGGTAGT
GGGATACGACGATACCGAAGACAGCTCATGTTATATCCCGCCGTTAACCACCATCAAACAGGATTTTCGCCTGCTGGGGCAAACCAGCGTGGACCGCTTGCTGCAACTCTCTCAG
GGCCAGGCGGTGAAGGGCAATCAGCTGTTGCCCGTCTCACTGGTGAAAAGAAAAACCACCCTGGCGCCCAATACGCAAACCGCCTCTCCCCGCGCGTTGGCCGATTCATTAATGC
AGCTGGCACGACAGGTTTCCCGACTGGAAAGCGGGCAGTGA
LacI_I12 Mutation (amino acid 3 is changed from CCA to TAT)
ATGAAATATGTAACGTTATACGATGTCGCAGAGTATGCCGGTGTCTCTTATCAGACCGTTTCCCGCGTGGTGAACCAGGCCAGCCACGTTTCTGCGAAAACGCGGGAAAAAGTGGAAGC
GGCGATGGCGGAGCTGAATTACATTCCCAACCGCGTGGCACAACAACTGGCGGGCAAACAGTCGTTGCTGATTGGCGTTGCCACCTCCAGTCTGGCCCTGCACGCGCCGTCGCAA
ATTGTCGCGGCGATTAAATCTCGCGCCGATCAACTGGGTGCCAGCGTGGTGGTGTCGATGGTAGAACGAAGCGGCGTCGAAGCCTGTAAAGCGGCGGTGCACAATCTTCTCGCGC
AACGCGTCAGTGGGCTGATCATTAACTATCCGCTGGATGACCAGGATGCCATTGCTGTGGAAGCTGCCTGCACTAATGTTCCGGCGTTATTTCTTGATGTCTCTGACCAGACACCC
ATCAACAGTATTATTTTCTCCCATGAAGACGGTACGCGACTGGGCGTGGAGCATCTGGTCGCATTGGGTCACCAGCAAATCGCGCTGTTAGCGGGCCCATTAAGTTCTGTCTCGGC
GCGTCTGCGTCTGGCTGGCTGGCATAAATATCTCACTCGCAATCAAATTCAGCCGATAGCGGAACGGGAAGGCGACTGGAGTGCCATGTCCGGTTTTCAACAAACCATGCAAATG
CTGAATGAGGGCATCGTTCCCACTGCGATGCTGGTTGCCAACGATCAGATGGCGCTGGGCGCAATGCGCGCCATTACCGAGTCCGGGCTGCGCGTTGGTGCGGATATCTCGGTAG
TGGGATACGACGATACCGAAGACAGCTCATGTTATATCCCGCCGTTAACCACCATCAAACAGGATTTTCGCCTGCTGGGGCAAACCAGCGTGGACCGCTTGCTGCAACTCTCTCA
GGGCCAGGCGGTGAAGGGCAATCAGCTGTTGCCCGTCTCACTGGTGAAAAGAAAAACCACCCTGGCGCCCAATACGCAAACCGCCTCTCCCCGCGCGTTGGCCGATTCATTAATG
CAGCTGGCACGACAGGTTTCCCGACTGGAAAGCGGGCAGTGA
LacI_X86 Mutation (amino acid 61 is changed from TCG to CTG)
ATGAAACCAGTAACGTTATACGATGTCGCAGAGTATGCCGGTGTCTCTTATCAGACCGTTTCCCGCGTGGTGAACCAGGCCAGCCACGTTTCTGCGAAAACGCGGGAAAAAGTGGAAGC
GGCGATGGCGGAGCTGAATTACATTCCCAACCGCGTGGCACAACAACTGGCGGGCAAACAGCTGTTGCTGATTGGCGTTGCCACCTCCAGTCTGGCCCTGCACGCGCCGTCGCAA
ATTGTCGCGGCGATTAAATCTCGCGCCGATCAACTGGGTGCCAGCGTGGTGGTGTCGATGGTAGAACGAAGCGGCGTCGAAGCCTGTAAAGCGGCGGTGCACAATCTTCTCGCGC
AACGCGTCAGTGGGCTGATCATTAACTATCCGCTGGATGACCAGGATGCCATTGCTGTGGAAGCTGCCTGCACTAATGTTCCGGCGTTATTTCTTGATGTCTCTGACCAGACACCC
ATCAACAGTATTATTTTCTCCCATGAAGACGGTACGCGACTGGGCGTGGAGCATCTGGTCGCATTGGGTCACCAGCAAATCGCGCTGTTAGCGGGCCCATTAAGTTCTGTCTCGGC
GCGTCTGCGTCTGGCTGGCTGGCATAAATATCTCACTCGCAATCAAATTCAGCCGATAGCGGAACGGGAAGGCGACTGGAGTGCCATGTCCGGTTTTCAACAAACCATGCAAATG
CTGAATGAGGGCATCGTTCCCACTGCGATGCTGGTTGCCAACGATCAGATGGCGCTGGGCGCAATGCGCGCCATTACCGAGTCCGGGCTGCGCGTTGGTGCGGATATCTCGGTAG
TGGGATACGACGATACCGAAGACAGCTCATGTTATATCCCGCCGTTAACCACCATCAAACAGGATTTTCGCCTGCTGGGGCAAACCAGCGTGGACCGCTTGCTGCAACTCTCTCA
GGGCCAGGCGGTGAAGGGCAATCAGCTGTTGCCCGTCTCACTGGTGAAAAGAAAAACCACCCTGGCGCCCAATACGCAAACCGCCTCTCCCCGCGCGTTGGCCGATTCATTAATG
CAGCTGGCACGACAGGTTTCCCGACTGGAAAGCGGGCAGTGA
LacI_I12_X86 Mutation
ATGAAATATGTAACGTTATACGATGTCGCAGAGTATGCCGGTGTCTCTTATCAGACCGTTTCCCGCGTGGTGAACCAGGCCAGCCACGTTTCTGCGAAAACGCGGGAAAAAGTGGAAGCGGCGATGGCGGAG
CTGAATTACATTCCCAACCGCGTGGCACAACAACTGGCGGGCAAACAGCTGTTGCTGATTGGCGTTGCCACCTCCAGTCTGGCCCTGCACGCGCCGTCGCAAATTGTCGCGGCGATTAAATCTCGCGCC
GATCAACTGGGTGCCAGCGTGGTGGTGTCGATGGTAGAACGAAGCGGCGTCGAAGCCTGTAAAGCGGCGGTGCACAATCTTCTCGCGCAACGCGTCAGTGGGCTGATCATTAACTATCCGCTGGATGAC
CAGGATGCCATTGCTGTGGAAGCTGCCTGCACTAATGTTCCGGCGTTATTTCTTGATGTCTCTGACCAGACACCCATCAACAGTATTATTTTCTCCCATGAAGACGGTACGCGACTGGGCGTGGAGCATC
TGGTCGCATTGGGTCACCAGCAAATCGCGCTGTTAGCGGGCCCATTAAGTTCTGTCTCGGCGCGTCTGCGTCTGGCTGGCTGGCATAAATATCTCACTCGCAATCAAATTCAGCCGATAGCGGAACGGG
AAGGCGACTGGAGTGCCATGTCCGGTTTTCAACAAACCATGCAAATGCTGAATGAGGGCATCGTTCCCACTGCGATGCTGGTTGCCAACGATCAGATGGCGCTGGGCGCAATGCGCGCCATTACCGAGT
CCGGGCTGCGCGTTGGTGCGGATATCTCGGTAGTGGGATACGACGATACCGAAGACAGCTCATGTTATATCCCGCCGTTAACCACCATCAAACAGGATTTTCGCCTGCTGGGGCAAACCAGCGTGGACC
GCTTGCTGCAACTCTCTCAGGGCCAGGCGGTGAAGGGCAATCAGCTGTTGCCCGTCTCACTGGTGAAAAGAAAAACCACCCTGGCGCCCAATACGCAAACCGCCTCTCCCCGCGCGTTGGCCGATTCAT
TAATGCAGCTGGCACGACAGGTTTCCCGACTGGAAAGCGGGCAGTGA
LacI Promoter
CGTTGACACCATCGAATGGCGCAAAACCTTTCGCGGTATGGCATGATAGCGCCCGG
LacIQ Promoter
CGTTGACACCATCGAATGGTGCAAAACCTTTCGCGGTATGGCATGATAGCGCCCGG
LacIQ1 Promoter
AGCGGCATGCATTTACGTTGACACCACCTTTCGCGGTATGGCATGATAGCGCCCGG
-These promoter sequences are taken from Glascock and Weickert 1998
| Part | Forward | Reverse |
|---|---|---|
| lacIQ1 Promoter | |

|
| lacIQ Promoter | 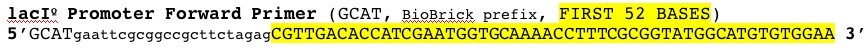 |

|
| LacI_I12 | 
| |
| LacI | |

|
| LacI_X86 (Primers for step 1 of PCR) | |

|
| LacI_I12X86 (Primers for step 1 of PCR) | |

|
| ### | ### | ### |