Designing XOR Gates - two campus approach
Contents
Davidson XOR Biological Design
Here is an idea Malcolm had.
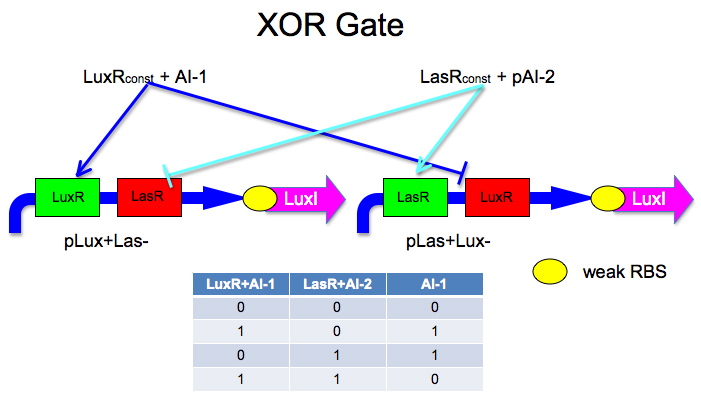
The idea is to have two mirrored halves of the system. One is regulated by AI-1 and the other by AI-2. There is a potential problem in that the Lux half is more likely to get positive feedback than the Las half. This MAY not be a problem because 0/0 is leaky so we put a weak RBS to minimize leaky protein production. Also, if we add AI-2 and AI-1 is produced by leak, then the entire system shuts down. The repressor site is located between -35 and -10 of the promoter. The activator binding site is upstream of -35. This has been documented by Egland and Greenberg
Missouri Western XOR Biological Design
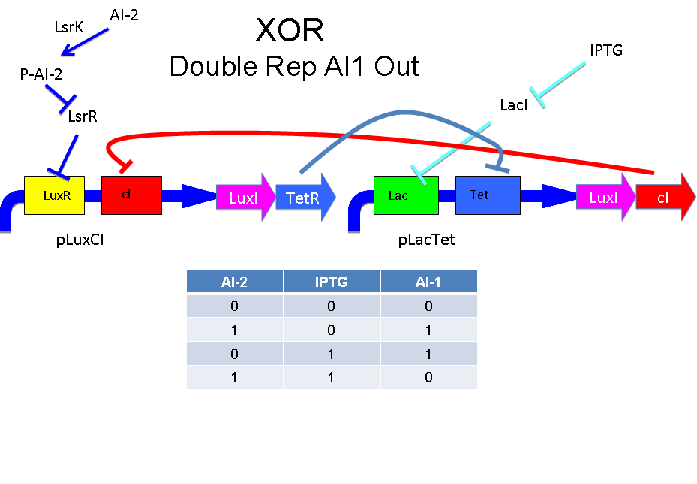
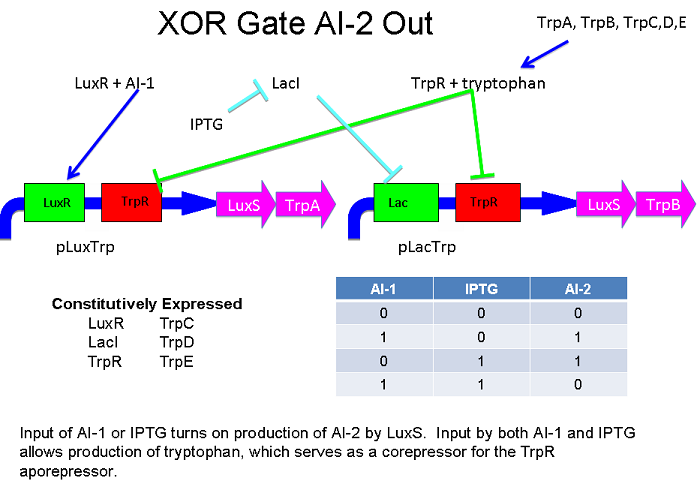
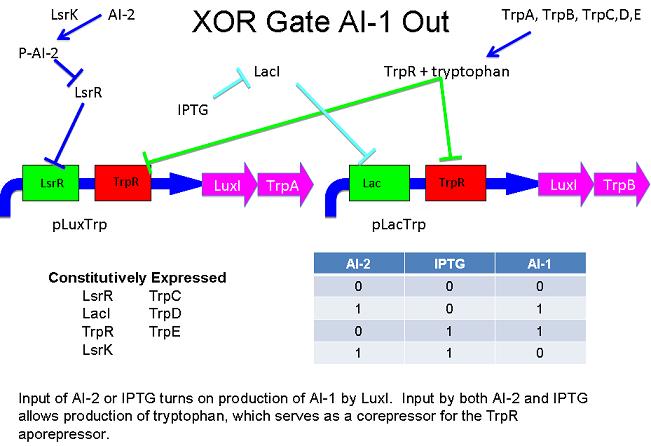
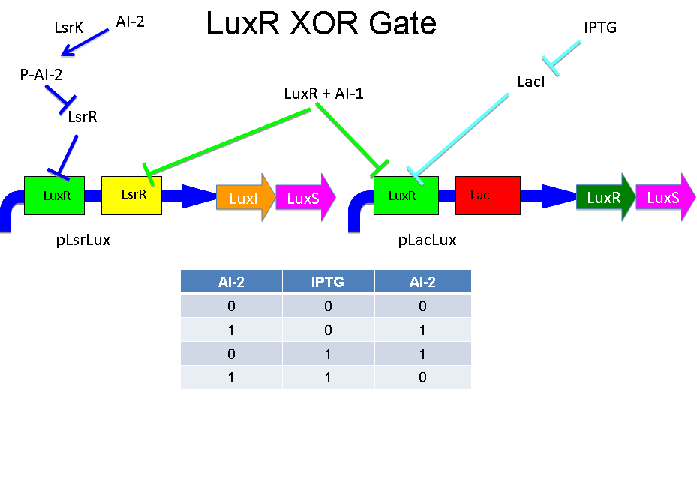
These two XOR circuits are designed to complement each other. Each recieves a cell-to-cell signal and a chemical signal and processes it into a cell-to-cell signal. Colonies that output AI-1 could alternate with colonies that produce AI-2.
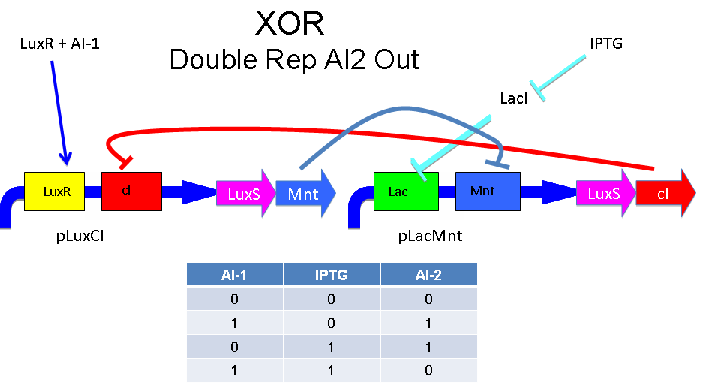
Above - Input of AI-2 or IPTG turns on production of AI-1 by LuxS. Input by both AI-2 and IPTG allows production of the repressors cI and TetR, which repress both transcription units. LsrK, LsrR and LacI are constitutively expressed.
Missouri Western XOR Biological Design
LacI could be the new LacI X86+I12.
Got it.
Also, the output gene should be LuxI, not LuxS. With LuxS, the second cell has to have the Las system in place of the Lux system.
JB/AMC
The idea here is that there are two different XOR gate clones. One takes input of AI1 and IPTG and outputs AI2. The other takes inputs of AI2 and IPTG and outputs AI2. These two clones could be alternated in a pathway of colonies. -TE/AG
XOR Based on Tryptophan Anabolism and the TrpR Repressor
Another XOR Biological Design
Davidson Ampicillin Communication: time delayed cell growth
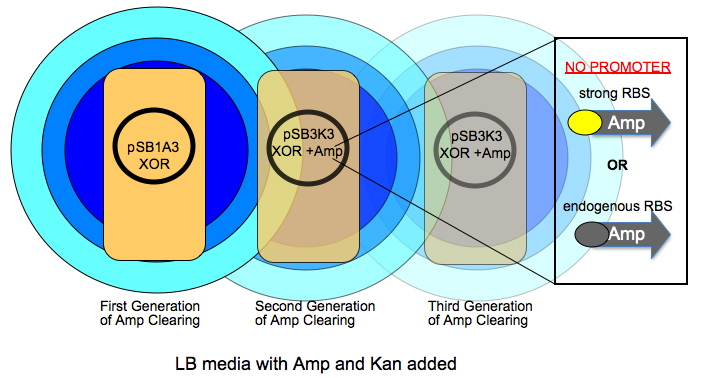
The very first colony has a high copy number plasmid that is Amp and Kan resistant (R). As this colony grows over time, it will digest ampicillin in an increasingly larger circle shown by radiating circles of blue. The subsequent colonies have a lower copy number plasmid and have a promoter-less version of AmpR in addition to the XOR construct. The AmpR coding will have either its native RBS or one we give it.
Davidson Growth Layouts
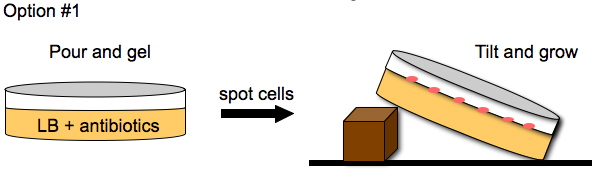
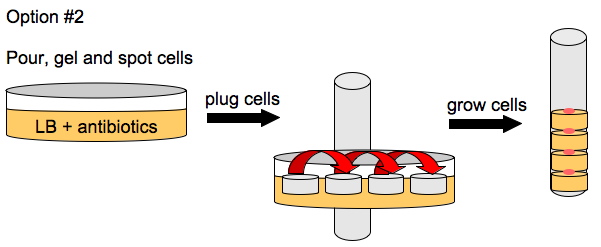
To enhance the unidirectional flow of AmpR, we could either grow the cells on a slant or create a vertical stack of agar plugs. The thickness of the plug would be determined by the thickness of the plates we pour. This may or may not help with the diffusion of AmpR but it is easier to do than microfluidics.