Difference between revisions of "Directed Evolution and Synthetic Biology - Hunter Stone"
(→Introduction - Optimization and Directed Evolution) |
(→The Theory of Directed Evolution) |
||
| Line 17: | Line 17: | ||
'''Figure 1:''' (Ro, 2006 - Permission Pending) | '''Figure 1:''' (Ro, 2006 - Permission Pending) | ||
| − | ==The | + | ==The Directed Evolution Method== |
One strategy that has the potential of addressing many of these questions within the context of synthetically constructed pathways is directed evolution. The directed evolution approach uses nature’s selective capabilities to test a large number of variants in a studied organism, pathway, or enzyme to find variants that confer greater efficiency. Directed evolution experiments follow these general steps: | One strategy that has the potential of addressing many of these questions within the context of synthetically constructed pathways is directed evolution. The directed evolution approach uses nature’s selective capabilities to test a large number of variants in a studied organism, pathway, or enzyme to find variants that confer greater efficiency. Directed evolution experiments follow these general steps: | ||
Revision as of 19:36, 2 December 2007
Contents
- 1 Project Proposal
- 2 Introduction - Optimization and Directed Evolution
- 3 The Directed Evolution Method
- 4 Proof of Concept: Directed Evolution vs. Rational Modeling in Lycopene-producing E. coli
- 5 Increases in Sophistication: Three Examples of Directed Evolution Showing Promise for Synthetic Biology
- 6 Works Cited
Project Proposal
My project will focus on attempts to utilize random mutations for optimization of synthetic pathways. Mathematical modeling of synthetic pathways is a powerful, proven tool to maximize product output. However, authors have recently shown that recombinant methods can be used to discover previously unknown elements of cell metabolism that will increase product yield even further. These methods of directed evolution have also been used to create powerful tools like promoters of specific expression levels, further increasing the relevance and importance of these methods to synthetic biology.
Introduction - Optimization and Directed Evolution
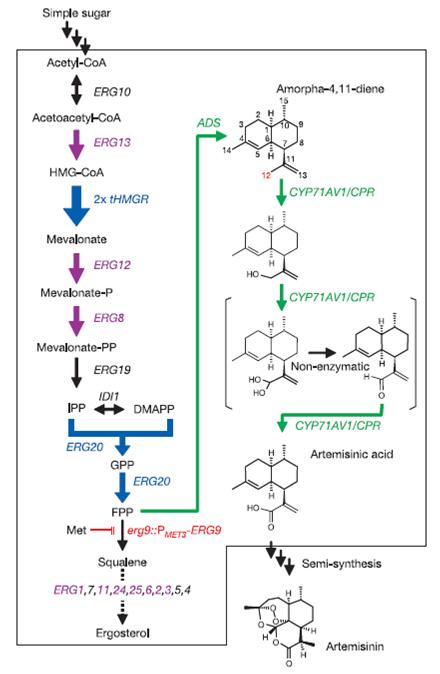
Researcher Jay Keasling has recently described a genetically-modified yeast strain that produces artemisinic acid, a chemical precursor to the antimalarial drug artemisinin. In these experiments, his team engineered yeast cells to express enzymes in a pathway that converts farnesyl pyrophosphate (FPP), a metabolic intermediate naturally occurring in yeast, into the desired product. Initially, however, this yeast strain was unable to produce any appreciable amount of artemisin. Keasling’s team had run into a key problem facing many projects in synthetic biology: optimization. Although we are increasingly able to build sophisticated constructs within living cells, the existence of these frameworks does not always correspond with their ability to fulfill their intended purposes efficiently and effectively.
Keasling’s team chose to address this problem by rationally modifying the metabolism of their yeast strain (Fig. 1). Although they were successful in increasing desired product yields, the authors note that further optimization is necessary to meet desired goals for cost of production. When one considers the work facing the group in the future, many questions arise. Were the changes they have already made to the yeast’s metabolism truly the best for meeting their goals? Could changes in other distantly-related metabolic pathways have helped to increase yields? Are there presently unknown elements in the cell affecting the new pathway which could potentially be changed? Are the enzymes themselves working at maximum efficiency?
One technique with the potential to answer all of these questions is directed evolution.
Figure 1: (Ro, 2006 - Permission Pending)
The Directed Evolution Method
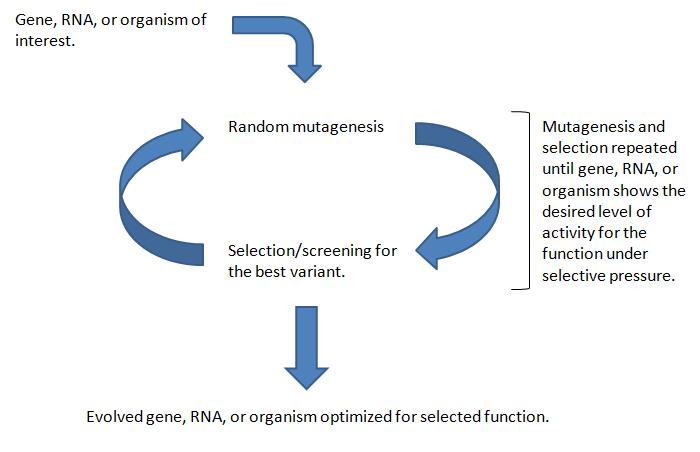
One strategy that has the potential of addressing many of these questions within the context of synthetically constructed pathways is directed evolution. The directed evolution approach uses nature’s selective capabilities to test a large number of variants in a studied organism, pathway, or enzyme to find variants that confer greater efficiency. Directed evolution experiments follow these general steps:
- A large library of variants of the targeted gene, pathway, or cell is generated using methods that randomly change genomic DNA, such as error-prone PCR or transposon integration.
- Screening or selection techniques specific for the function of interest (e.g. higher enzyme efficiency, greater cell resistance to ethanol) reveal the most productive members of the variant library.
- The most productive variant is resubmitted to the genetic randomization and selection processes until the desired result is received - an evolved mutant more adept at the processes it was selected for than its wild-type parent.
One cycle of genomic randomization and selection is called a round.
The power of directed evolution comes from two sources: its nonbiased nature and its capability of probing regions of the cell currently undescribed or beyond the present capabilities of linear modeling and reasoning.
For a retorspective review of the technique, click here.
Proof of Concept: Directed Evolution vs. Rational Modeling in Lycopene-producing E. coli
Both of these ideas were recently demonstrated in the work of researchers Hal Alper and Greg Stephanopoulos. Previously, the two researchers had created a strain of E. coli capable of synthesizing lycopene, a carotenoid which naturally occurs in tomatoes but has more recently been incorporated into vitamin tablets for its antioxidant capabilities. The team was interested in how two different sets of gene knockouts, one predicted by computer modeling and the other selected through directed evolution tests, compared in their ability to increase this strain’s lycopene production. They were also interested in whether these two types of gene knockouts would have additive effects and increase lycopene production more if they were expressed together.
To answer both of these questions, the researchers first used a previously described computer model to identify eight gene knockouts that were predicted to increase lycopene production. They complimented these experiments with an in vivo directed evolution test to indentify a second set of knockout sites. This test used transposon integration to create a library of random genome-wide gene knockout strains. The best of these knockout strains were selected through plating, which revealed lycopene production efficiency as a function of red colony color. This directed evolution test selected three gene knockout sites.
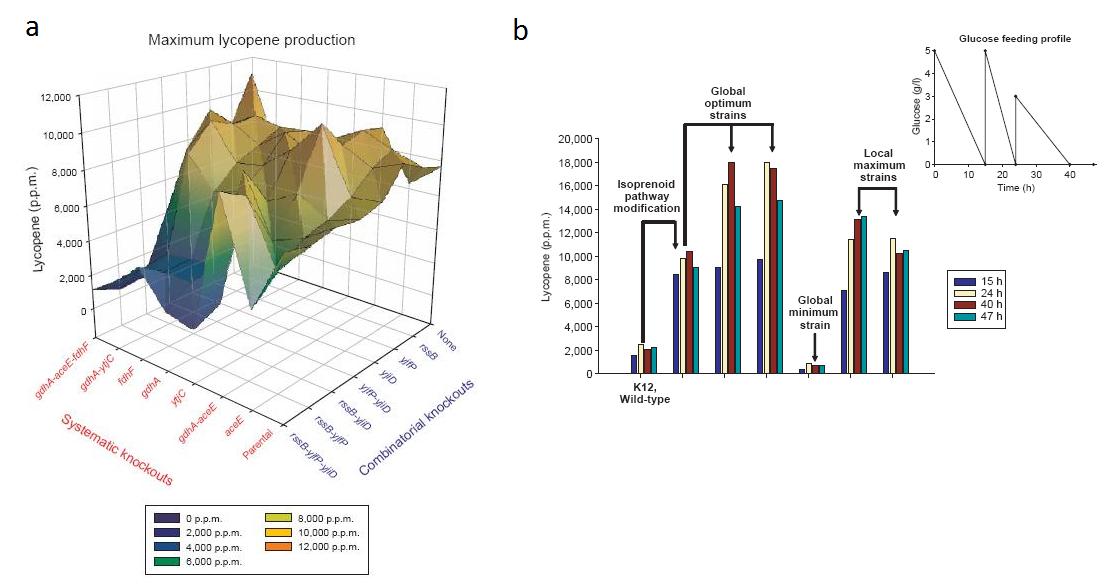
By combining all permutations of these three knockouts (one, two, and three gene knockouts) and their parental strain with the eight model-predicted knockouts in all possible ways, the researchers created 64 unique strains of bacteria to examine how randomly selected and systematically predicted gene knockouts might interact to increase lycopene production. Of the two maximum lycopene-producing strains (measured through absorbance of extracted lycopene at 475 nm), one strain had a knockout selected through directed evolution testing (Fig. 2a). Furthermore, this particular knockout strain also showed an earlier peak in lycopene production when compared to the completely stystematically-predicted knockout strain in batch fed culture (Fig. 2b).
(Alper, 2005 - Permission Pending)
Figure 2: The two measurements lycopene production in knockout strains of lycopene producing bacteria. (a) A landscape displaying the 64 strains resulting for all possible combinations of gene knockouts selected through systematic modeling and directed evolution (combinatorial knockouts). Lycopene production for each strain was measured at the end of a 48-h shake-flask fermentation and amount of lycopene produced was quantified through extraction form the cell pellet with acetone and supernatant absorbance at 475 nm. Of interest is global maximum strain ΔgdhA ΔaceE ΔPyjiD, which contains a knockout of the ΔPyjiD gene selected through directed evolution testing. (b) Lycopene production of the best knockout strains in batch-fed culture. From left to right, the K12 strain from which combinatorial mutants were derived, the preengineered parental strain from which the systematically-selected knockout strains were derived, global maximum strain ΔgdhA ΔaceE ΔfdhF, global maximum strain ΔgdhA ΔaceE ΔPyjiD, and the global minimum strain and two local maximum strains from landscape 1a. Of interest is knockout strain ΔgdhA ΔaceE ΔPyjiD (fourth from the left). This strain, which has a gene knockout selected through directed evolution testing, shows the same maximum in lycopene productivity as the entirely systematically-predicted strain ΔgdhA ΔaceE ΔfdhF (third from left), but also shows an earlier peak in this productivity and more sustained lycopene production.
Increases in Sophistication: Three Examples of Directed Evolution Showing Promise for Synthetic Biology
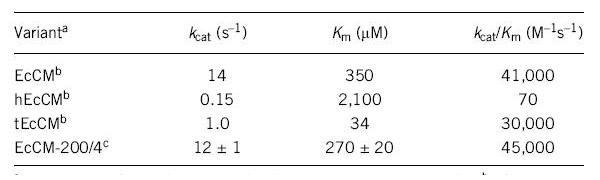
Neuenschwander, M., M. Butz, C. Heintz & D. Hilvert. 2007. A simple selection strategy for evolving highly efficient enzymes. Nature Biotechnology 25(10): 1145-1147.
(Neuenschwander, 2007 - Permission Pending)
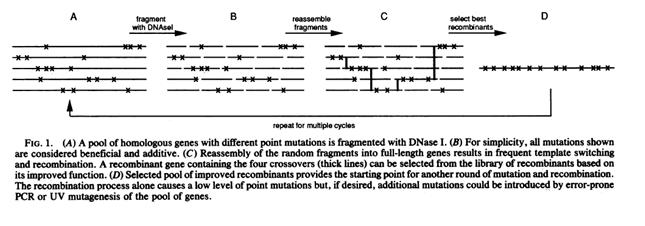
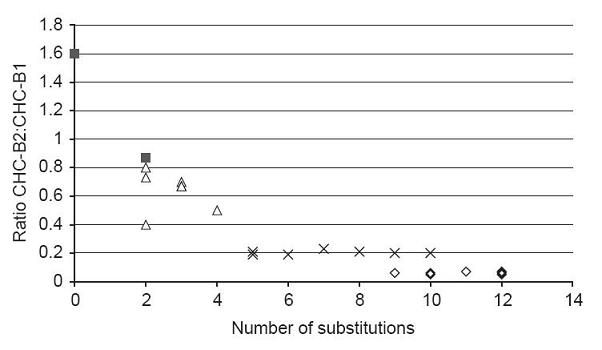
Stutzman-Engwall, K., S. Conlon, R. Fedechko, H. McArthur, K. Pekrun, Y. Chen, S. Jenne, C. La, N. Trinh, S. Kim, Y. Zhang, R. Fox, C. Gustafsson & A. Krebber. 2005. Semi-synthetic DNA shuffling of aveC leads to improved industrial scale production of doramectin by Streptomyces avermitilis. Metabolic Engineering 7: 27-37.
(Stemmer, 1994 - Permission Pending)
(Stutzman-Engwall, 2005 - Permission Pending)
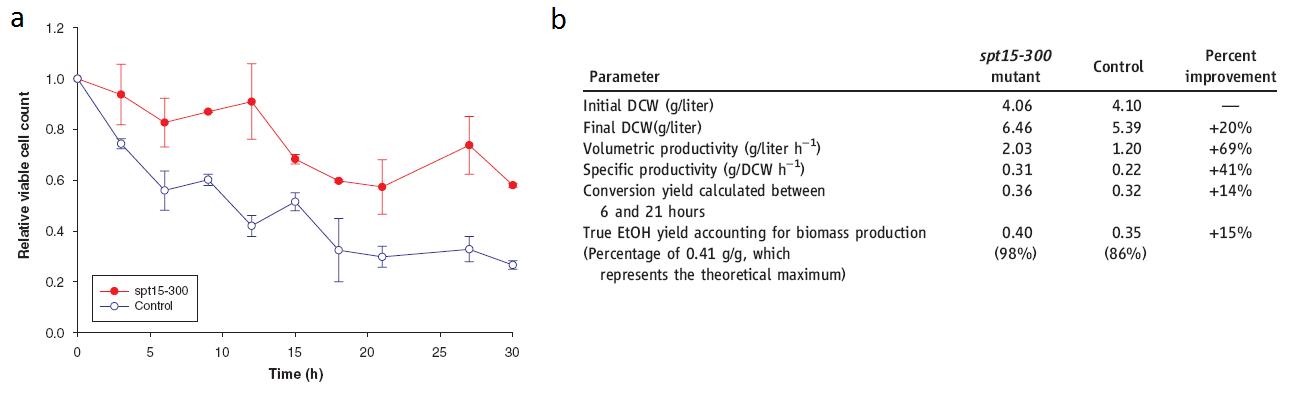
Alper, H., J. Moxley, E. Nevoigt, G.R. Fink & G. Stephanopoulos. 2006. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314: 1565-1568.
(Alper, 2006 - Permission Pending)