Directed Evolution and Synthetic Biology - Hunter Stone
Contents
Project Proposal
My project focuses on the use of random mutations to optimize synthetic pathways. Mathematical modeling of synthetic pathways is a powerful, proven tool to maximize product output. However, recently a series of unbiased strategies using recombinant methods have been shown to further increase product yield. These methods, which has been referred to as directed evolution, have produced powerful new methods and approaches for the synthetic biologist.
Introduction - Pathway Optimization and Directed Evolution
Researcher Jay Keasling has recently described a genetically-modified yeast strain that produces artemisinic acid, a chemical precursor to the antimalarial drug artemisinin. In these experiments, his team engineered yeast cells to express enzymes in a pathway that converts farnesyl pyrophosphate (FPP), a metabolic intermediate naturally occurring in yeast, into the desired product. Initially, however, this yeast strain was unable to produce any appreciable amount of artemisin. Keasling’s team had run into a key problem facing many projects in synthetic biology: optimization. Although we are increasingly able to build sophisticated constructs within living cells, the existence of these frameworks does not always correspond with the ability to fulfill their intended purposes efficiently and effectively.
Keasling’s team chose to address this problem by rationally modifying the metabolism of their yeast strain. Although they were successful in increasing desired product yields, the authors note that further optimization is necessary to meet their goals for cost of production. When one considers the work facing the group in the future, many questions arise. Were the changes they have already made to the yeast’s metabolism truly the best for optimizing artemisinin output? Could changes in other distantly-related metabolic pathways have also helped to increase yields? Are there presently unknown elements in the cell affecting the new pathway which could potentially be changed? Are the enzymes in the new pathway themselves working at maximum efficiency?
One technique with the potential to answer all of these questions is directed evolution.
Directed Evolution: The Method
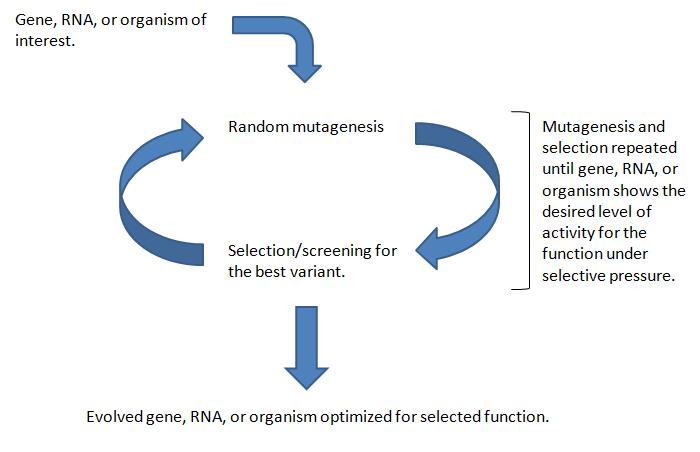
Directed evolution is a method used to create a more efficient mutant of an existing gene, RNA, pathway or cell. The method follows these general steps:
- A library of variants of the targeted construct (e.g. a gene, a cell, etc.) is generated through random changes of its genomic DNA. Methods of genetic randomization include error-prone PCR, mutagenic agents like Mutazyme, or random transposon integration.
- The variant library goes through a process of screening or selection to reveal the most productive members of the library. Selection and screening techniques are specific to desired function of each experiment (e.g. higher enzyme efficiency, greater cell resistance to ethanol).
- The most productive variant is resubmitted to the genetic randomization and selection processes.
- Steps 1-3 are repeated until the desired result is received - an evolved mutant more adept at the processes it was selected for than its unevolved parent.
Directed Evolution and Synthetic Biology
The power of directed evolution comes from two sources: its nonbiased nature and its ability to test changes in elements of the cell beyond present knowledge and understanding. The method has historically been used to maximize the function of a particular protein . New methods have recently been developed to maximize the function not just of a single protein, but of more complex phenotypes.
Optimization of Protein Function and Yield
Many projects in synthetic biology involve introducing foreign enzymatic pathways into microbes. Examples include yeast cells capable of producing atremisinin (Ro et al., 2006) and current attempts by a number of companies to engineer microbes producing fossil fuels (Amyris, LS9). The quantity of output from these pathways ultimately depends on the efficiency of their enzymes. However, rational reengineering these enzymes is an extremely difficult task due to the complexities of protein structure. In addition, such work would require a wealth of primary research about the enzyme of interest to do correctly.
The following two papers describe successful efforts at using directed evolution to improve enzymes in pathways aimed at product formation. In both, the authors are able to circumvent the laborious task of rational protein engineering through using directed evolution to meet their goals. In addition, both papers describe significant improvements to the methodology of directed evolution itself. The authors of the first paper enhance the technique by improving the process of genetic randomization, while the authors of the second paper achieve the same goal by improving selection methods.
One can envision using similar methods for improvement of individual enzymes in a synthetically-engineered microbe.
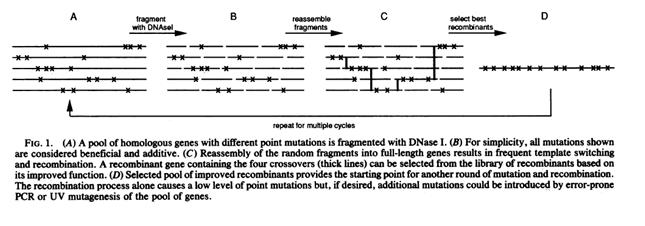
Semi-Synthetic DNA Shuffling and Doramectin
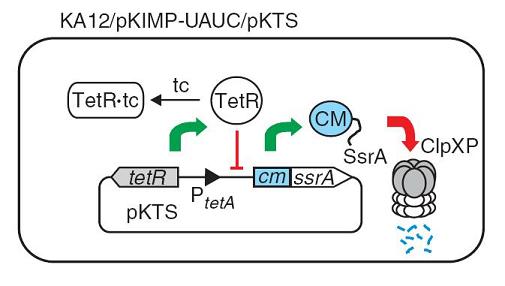
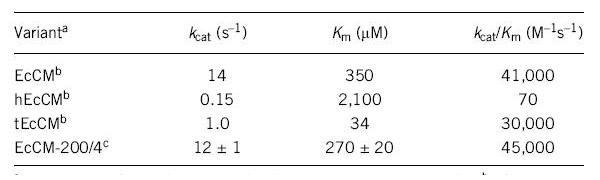
A Simple Method for Highly Evolved Enzymes
"Genome-wide" Directed Evolution
A second, emerging branch of directed evolution attempts to improve phenotypes regulated not just by an individual gene but by multiple genes across the entire genome.
This type of directed evolution could be invaluable to synthetic biology by providing methods to test how parts work together as well how they work in the cell.
The following two papers describe attempts to use "genome-wide" directed evolution to optimize organisms for a specific phenotype. In the first, Alper et al. use random gene knockouts in concert with model-predicted knockouts to optimize the output of a lycopene-producing strain of E. coli. In the second, the authors engineer a strain of yeast with high levels of ethanol production and resistance. This paper also describes a promising new method which allowed the team to circumvent rational modeling altogether in the creation of this strain of yeast.
Gene Knockout for Lycopene Overproduction
Global Transcriptome Machinery Engineering
Conclusion
Figure Bank
Neuenschwander, M., M. Butz, C. Heintz & D. Hilvert. 2007. A simple selection strategy for evolving highly efficient enzymes. Nature Biotechnology 25(10): 1145-1147.
(Neuenschwander, 2007 - Permission Pending)
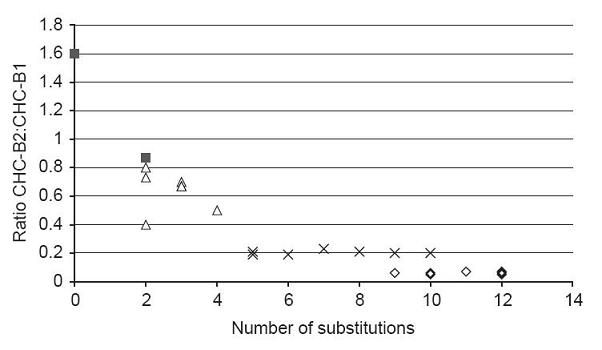
Stutzman-Engwall, K., S. Conlon, R. Fedechko, H. McArthur, K. Pekrun, Y. Chen, S. Jenne, C. La, N. Trinh, S. Kim, Y. Zhang, R. Fox, C. Gustafsson & A. Krebber. 2005. Semi-synthetic DNA shuffling of aveC leads to improved industrial scale production of doramectin by Streptomyces avermitilis. Metabolic Engineering 7: 27-37.
(Stemmer, 1994 - Permission Pending)
(Stutzman-Engwall, 2005 - Permission Pending)
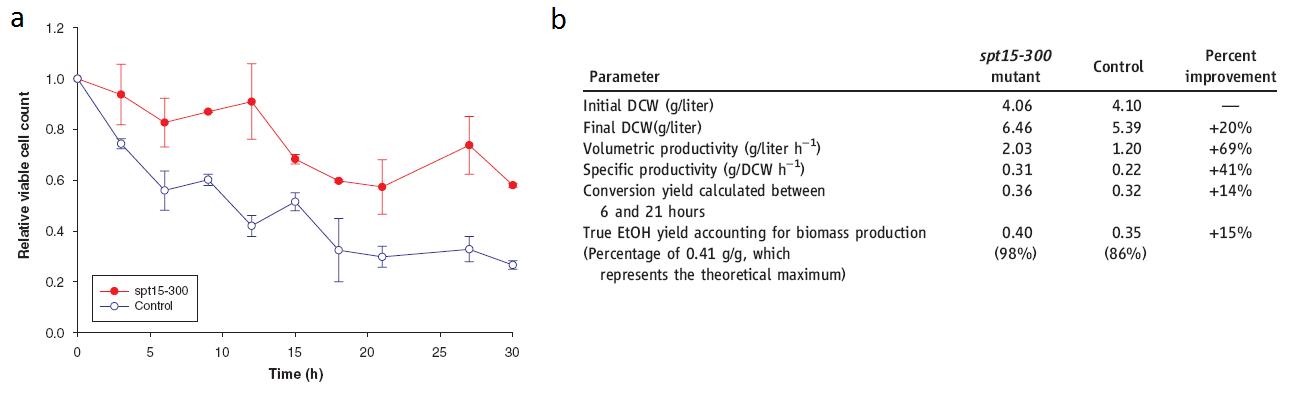
Alper, H., J. Moxley, E. Nevoigt, G.R. Fink & G. Stephanopoulos. 2006. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314: 1565-1568.
(Alper, 2006 - Permission Pending)