Lauren
Natural Drugs; Flavonoids and Stilbenes
Flavonoids are polyphenolic biochemical compounds that have been shown to have antioxidant effects. They are known to be found in fruits, vegetable, olive oil, cocoa and beverages such as tea and red wine.There are over 4000 known flavonoids subdivided based on chemical structure. [1] The most common flavonoids include anthocyanins, flavols, flavones, flavanones, flavan-3-ols, and isoflavones.
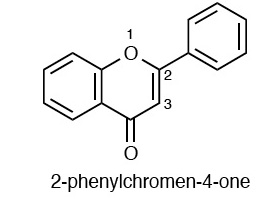 <--- general Flavonoid structures --->
<--- general Flavonoid structures ---> 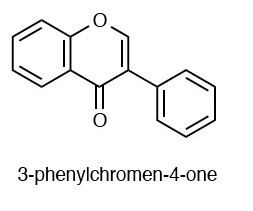 [2]
[2]
One subgroup of flavonoids called anthocyanins, are found in the pigments of various foods and are most known for their health benefits. The anthocyanins may have benefits such as protection against DNA cleavage and enzyme inhibition, and increased cellular immune response and membrane strength. [3]
Stilbenes, like Flavonoids, are polyphenolic compounds have been the focus of clinical research for cancer prevention. [4] One of the most commonly known stilbene, resveratrol, has been shown to have anticancer properties and the ability to suppress proliferation of cancer cells.[5]
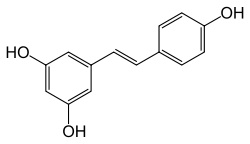 <--- Resveratrol structure [6]
<--- Resveratrol structure [6]
Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals
inhibition of mitochondrial F0F1-ATPase/ATP synthase could be a potential mechanism contributing to the many effects of dietary polyphenolics. It has been shown that the same ATP synthase is localized also on the plasma membranes of several tumour cell lines (Das et al., 1994) and human umbilical vein endothelial cells, and that it is a binding protein for angiostatin, a potent protein inhibitor of angiogenesis and cell proliferation (Moser et al., 1999). Therefore, phytoestrogens could also play a similar role by binding the ATP synthase on the plasma membrane. In fact, genistein has been shown to be a potent inhibitor of angiogenesis (Fotsis et al., 1993). Moreover, resveratrol and genistein also inhibit the bovine mitochondrial NADH:ubiquinone oxidoreductase and induce ornithine decarboxylase, a marker for cancer chemopreventive potency, with IC50=30μM (Fang & Casida, 1998). The simultaneous effects of these phytochemicals on NADH:ubiquinone oxidoreductase and F0F1-ATPase/ATP synthase could significantly affect mitochondrial function and alter ATP level, mitochondrial transmembrane potential and generation of reactive oxygen species, which have been implicated in many cellular processes such as cellular protection, apoptosis, O2 sensing and ageing.[7]
Acid invertase
Invertase (EC 3.2.1.26 ) (systematic name: beta-fructofuranosidase) is an enzyme that catalyzes the hydrolysis (breakdown) of sucrose (table sugar). The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose. Invertases cleave the O-C(fructose) bond, whereas the sucrases cleave the O-C(glucose) bond.[1]
AI appears to be the key enzyme induced by root restriction that explains the higher sugar content found in grape berry produced under root restriction.
The characteristic rapid rise in the rate of sugar accumulation by the berries about halfway through development was preceded by an increase in the activity of invertase.
In fact, the protein fraction retrieved from a buffered medium after incubation of ripening berry slices contained a soluble invertase of presumably vacuolar origin with an acid pH-activity profile and a pI of about 4. [9]
These observations have led to speculations that suc hydrolysis primarily by acid invertase may determine the rate and extent of SUC storage in tomato fruit (Walker et al., 1978). In contrast, low levels of acid invertase are associated with high levels of SUC accumulation in L. hirsutum (Miron
The levels of invertase activity were found to vary significantly among 14 varieties of grapes tested. The crude invertases were, however, similar in both pH and temperature optima, as well as pH and thermal stabilities. The enzymes showed a high degree of thermostability and were also stable at acidic pH and high concentration of alcohol. A significant level of invertase activity persisted in several white table wines. The optimum activity of the enzyme purified from Semillon grape was observed at about 75°C and it was stable up to 70°C. Although the enzyme was stable between the pH 2 and 8, the optimum pH for its activity was about 4. The enzyme, whose molecular weight was estimated to be 65,000 by SDS polyacrylamide slab gel electrophoresis, was found to be a glycoprotein with a total carbohydrate content of 33%. This enzyme showed activity toward sucrose and raffinose, but was inactive on the other disaccharides tested. [10]