Difference between revisions of "Protein Export"
MaCampbell (talk | contribs) (→Proteins Exported Completely) |
MaCampbell (talk | contribs) (→Proteins Exported Completely) |
||
| (23 intermediate revisions by the same user not shown) | |||
| Line 16: | Line 16: | ||
http://www.membranetransport.org/ | http://www.membranetransport.org/ | ||
| − | '''1. Sec pathway''' using SecA, | + | '''1. Sec pathway''' using SecA, '''SecD''', '''SecE''', '''SecY''', SecC, '''SecF''' proteins.<br> |
*Our genome does not have any SecA annotations. | *Our genome does not have any SecA annotations. | ||
| + | *Our genome has 2500587981 HutaDRAFT_03460 Preprotein translocase subunit SecD | ||
| + | *Our genome has 2500589223 HutaDRAFT_15880 protein translocase subunit secE/sec61 gamma | ||
| + | *Our genome has >2500590667 HutaDRAFT_30320 protein translocase subunit secY/sec61 alpha | ||
| + | *Our genome does not have SecC annotations | ||
| + | *Our genome has >2500587980 HutaDRAFT_03450 protein translocase subunit secF | ||
SPaseI and II cleave peptides off for export. Sec11a and sec11b have been found in halophiles. | SPaseI and II cleave peptides off for export. Sec11a and sec11b have been found in halophiles. | ||
| + | Our annotations found: | ||
| + | *2500589648 HutaDRAFT_20130 signal peptidase I, archaeal type | ||
| + | *2500589949 HutaDRAFT_23140 Signal peptidase I [Halorhabdus utahensis AX-2, DSM 12940] | ||
| + | *2500589950 HutaDRAFT_23150 Signal peptidase I [Halorhabdus utahensis AX-2, DSM 12940] | ||
| + | *complement(2015867..2016427) "Signal peptidase I (EC 3.4.21.89) | ||
| − | + | Our genome has other required proteins: | |
| − | + | *2500587797 HutaDRAFT_01620 signal recognition particle, subunit SRP19 (srp19) | |
| + | *2500589597 HutaDRAFT_19620 signal recognition particle subunit FFH/SRP54 (srp54) | ||
| + | *2500589664 HutaDRAFT_20290 signal recognition particle-docking protein FtsY | ||
| − | '''2. Tat pathway''' using TatA, TatCo, TatCt, TatAo TatAt, TatB, TorD and TorA are chaperones that bind to signal sequences bound for Tat protein export. | + | '''2. Tat pathway''' using '''TatA''', TatCo, TatCt, TatAo TatAt, TatB, TorD and TorA are chaperones that bind to signal sequences bound for Tat protein export. |
Our Organism has these genes:<br> | Our Organism has these genes:<br> | ||
*ORF01012 sec-independent protein translocase component TatA 1 | *ORF01012 sec-independent protein translocase component TatA 1 | ||
| + | *2500588590 HutaDRAFT_09550 twin arginine-targeting protein translocase, TatA/E family | ||
*No TatCo in annotations | *No TatCo in annotations | ||
| − | * | + | *2500589362 HutaDRAFT_17270 Twin arginine targeting (Tat) protein translocase TatC |
| + | *2500589363 HutaDRAFT_17280 Twin arginine targeting (Tat) protein translocase TatC/Twin arginine targeting (Tat) protein translocase TatC, Archaeal clade | ||
*No TatAo in annotations | *No TatAo in annotations | ||
*No TatAt in annotations | *No TatAt in annotations | ||
| + | *No TatB in annotations | ||
| + | *No TorD in annotations | ||
| + | *No TorA in annotations | ||
| − | Tat proteins use protein domain of SRRXFLK | + | Tat proteins use protein domain of SRRXFLK to be targeted to be exported, I found in a hand search many proteins, but a short list is here: |
| + | *ORF00044 cellulase (glycosyl hydrolase family 5), putative - MTDPDRPPTGDREASQSNTTTGGEGP'''SRRTFLK'''...<br> | ||
| + | *2500587682 HutaDRAFT_00470 phage tail protein, P2 protein I family - MTRRTNDTGEVDEKPSSGAEQQGSNDSTGSRDP'''SRRDFLK'''... | ||
| + | *ORF00047 CHU large protein; candidate polyfunctional acetylxylan esterase-B-xylosidase-a-L-arabinofuranosidase, CBM9 module, Glycoside Hydrolase Family 43 protein and Carbohydrate Esterase Family 6 protein - MTRRTNDTGEVDEKPSSGAEQQGSNDSTGSRDP'''SRRDFLK'''... | ||
| + | *2500587687 HutaDRAFT_00520 Endoglucanase - MTHNNPDDDSTARRTTESTESPSTAGIASA'''SRRDFLK'''... | ||
| + | *ORF00053 exoglucanase A (Exocellobiohydrolase A) (1,4-beta-cellobiohydrolase A) (CBP95) [3.2.1.91] - MTHNNPDDDSTARRTTESTESPSTAGIASAS'''RRDFLK'''... | ||
| + | *2500587690 HutaDRAFT_00550 Cellulase (glycosyl hydrolase family 5)./Fibronectin type III domain./Carbohydrate binding module (family 6). MTDEATESIEASATDHTDETAGNRKDPGLT'''SSRRTFLG'''... not identical to consensus, but close. | ||
| + | |||
| + | |||
| + | They transport fully folded proteins. Many halophiles have a lot of K+ in cytoplasm to counter balance extracellular salt. Their proteins have many negative amino acids that help keep the proteins and bacteria from “salting out” and may lead to rapid protein folding. Therefore, the Tat system may be more important than the Sec system. Figure from Sonja-Verena Albers, Zalán Szabó and Arnold J. M. Driessen. 2006. Nature Reviews: Microbiology. VOLUME 4. | ||
<center> | <center> | ||
[[Image:Tat.png]] | [[Image:Tat.png]] | ||
| Line 42: | Line 68: | ||
Type I – V secretion systems possible. | Type I – V secretion systems possible. | ||
I is ABC protein transporter | I is ABC protein transporter | ||
| + | Our genome has: | ||
| + | *ORF00738 type II-IV secretion system proteins VirB11-TadA | ||
Flagella and pilus or pili proteins. | Flagella and pilus or pili proteins. | ||
PibD trims off cytoplasmic parts of flagellar proteins | PibD trims off cytoplasmic parts of flagellar proteins | ||
Latest revision as of 16:15, 18 November 2008
Proteins On the Cell Surface
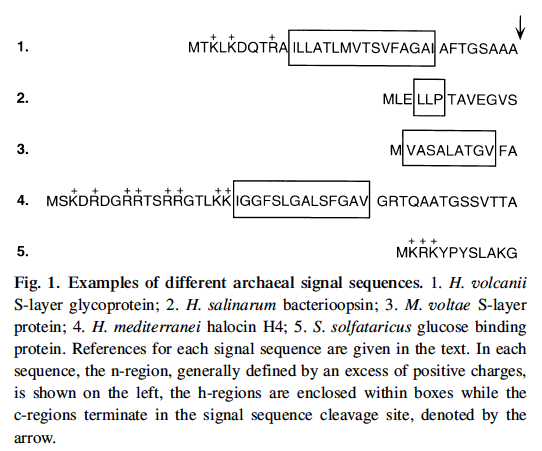
First, we should briefly consider proteins that reside in cell membrane. Like all other organisms, Halophile proteins begin with a signal sequence (20 - 30 residues long) that has many positive charges on it. We do not know enough to search for these in a reliable way. See five examples in the figure below from a review paper by Jerry Eichler, 2000. Archaeal protein translocation: Crossing membranes in the third domain of life. Eur. J. Biochem. 267: 3402-3412.
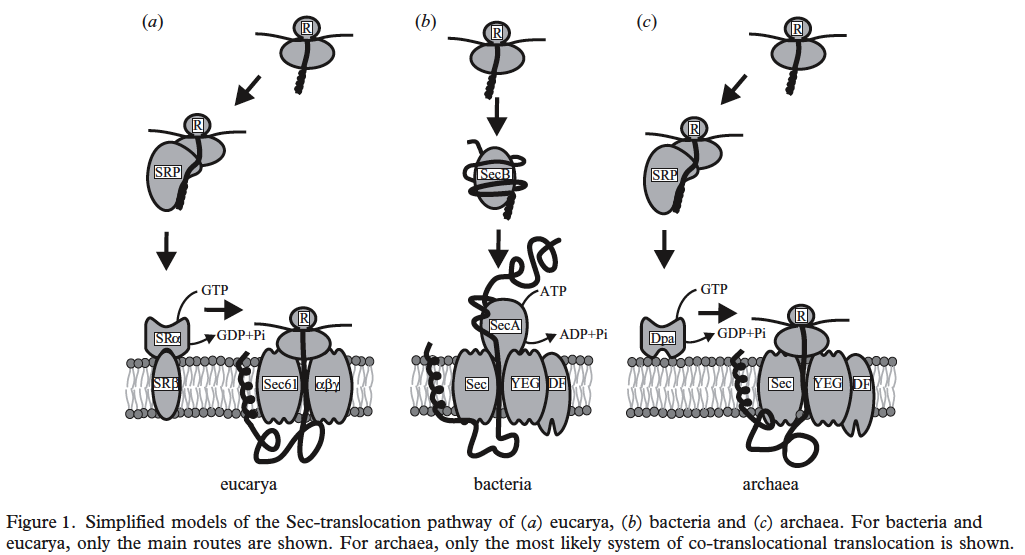
Three-domain comparison from Albert Bolhuis. 2004. The archaeal Sec-dependent protein translocation pathway. Phil. Trans. R. Soc. Lond. B. Vol. 359: 919–927.
Proteins Exported Completely
There are three main systems for protein export.
http://www.membranetransport.org/
1. Sec pathway using SecA, SecD, SecE, SecY, SecC, SecF proteins.
- Our genome does not have any SecA annotations.
- Our genome has 2500587981 HutaDRAFT_03460 Preprotein translocase subunit SecD
- Our genome has 2500589223 HutaDRAFT_15880 protein translocase subunit secE/sec61 gamma
- Our genome has >2500590667 HutaDRAFT_30320 protein translocase subunit secY/sec61 alpha
- Our genome does not have SecC annotations
- Our genome has >2500587980 HutaDRAFT_03450 protein translocase subunit secF
SPaseI and II cleave peptides off for export. Sec11a and sec11b have been found in halophiles. Our annotations found:
- 2500589648 HutaDRAFT_20130 signal peptidase I, archaeal type
- 2500589949 HutaDRAFT_23140 Signal peptidase I [Halorhabdus utahensis AX-2, DSM 12940]
- 2500589950 HutaDRAFT_23150 Signal peptidase I [Halorhabdus utahensis AX-2, DSM 12940]
- complement(2015867..2016427) "Signal peptidase I (EC 3.4.21.89)
Our genome has other required proteins:
- 2500587797 HutaDRAFT_01620 signal recognition particle, subunit SRP19 (srp19)
- 2500589597 HutaDRAFT_19620 signal recognition particle subunit FFH/SRP54 (srp54)
- 2500589664 HutaDRAFT_20290 signal recognition particle-docking protein FtsY
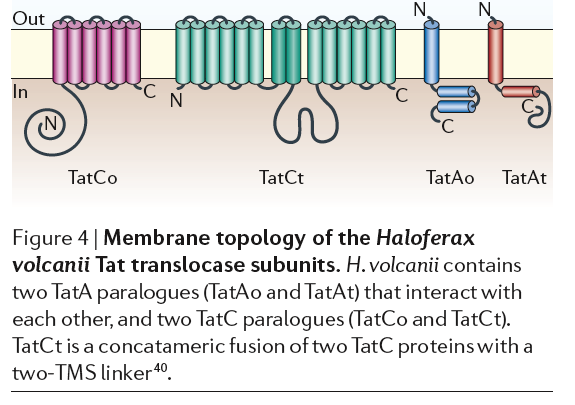
2. Tat pathway using TatA, TatCo, TatCt, TatAo TatAt, TatB, TorD and TorA are chaperones that bind to signal sequences bound for Tat protein export.
Our Organism has these genes:
- ORF01012 sec-independent protein translocase component TatA 1
- 2500588590 HutaDRAFT_09550 twin arginine-targeting protein translocase, TatA/E family
- No TatCo in annotations
- 2500589362 HutaDRAFT_17270 Twin arginine targeting (Tat) protein translocase TatC
- 2500589363 HutaDRAFT_17280 Twin arginine targeting (Tat) protein translocase TatC/Twin arginine targeting (Tat) protein translocase TatC, Archaeal clade
- No TatAo in annotations
- No TatAt in annotations
- No TatB in annotations
- No TorD in annotations
- No TorA in annotations
Tat proteins use protein domain of SRRXFLK to be targeted to be exported, I found in a hand search many proteins, but a short list is here:
- ORF00044 cellulase (glycosyl hydrolase family 5), putative - MTDPDRPPTGDREASQSNTTTGGEGPSRRTFLK...
- 2500587682 HutaDRAFT_00470 phage tail protein, P2 protein I family - MTRRTNDTGEVDEKPSSGAEQQGSNDSTGSRDPSRRDFLK...
- ORF00047 CHU large protein; candidate polyfunctional acetylxylan esterase-B-xylosidase-a-L-arabinofuranosidase, CBM9 module, Glycoside Hydrolase Family 43 protein and Carbohydrate Esterase Family 6 protein - MTRRTNDTGEVDEKPSSGAEQQGSNDSTGSRDPSRRDFLK...
- 2500587687 HutaDRAFT_00520 Endoglucanase - MTHNNPDDDSTARRTTESTESPSTAGIASASRRDFLK...
- ORF00053 exoglucanase A (Exocellobiohydrolase A) (1,4-beta-cellobiohydrolase A) (CBP95) [3.2.1.91] - MTHNNPDDDSTARRTTESTESPSTAGIASASRRDFLK...
- 2500587690 HutaDRAFT_00550 Cellulase (glycosyl hydrolase family 5)./Fibronectin type III domain./Carbohydrate binding module (family 6). MTDEATESIEASATDHTDETAGNRKDPGLTSSRRTFLG... not identical to consensus, but close.
They transport fully folded proteins. Many halophiles have a lot of K+ in cytoplasm to counter balance extracellular salt. Their proteins have many negative amino acids that help keep the proteins and bacteria from “salting out” and may lead to rapid protein folding. Therefore, the Tat system may be more important than the Sec system. Figure from Sonja-Verena Albers, Zalán Szabó and Arnold J. M. Driessen. 2006. Nature Reviews: Microbiology. VOLUME 4.
3. lipobox pathway for lipid-modified proteins, lipobox = [I/L/G/A][A/G/S]"cut_here"C is the target for SPaseII enzymes.
Type I – V secretion systems possible. I is ABC protein transporter Our genome has:
- ORF00738 type II-IV secretion system proteins VirB11-TadA
Flagella and pilus or pili proteins. PibD trims off cytoplasmic parts of flagellar proteins