Riboregulators
All information on riboregulators came from Isaacs et al. 2004.
After observing the many and varied naturally occuring post-transcriptional, regulatory RNA systems, Isaacs et al. designed and engineered a modular synthetic system where RNA turns on and off gene expression by controlling translation. The modularity of their system allows any gene to be regulated instead of only a specific gene to which the riboregulator is targeted.
Design
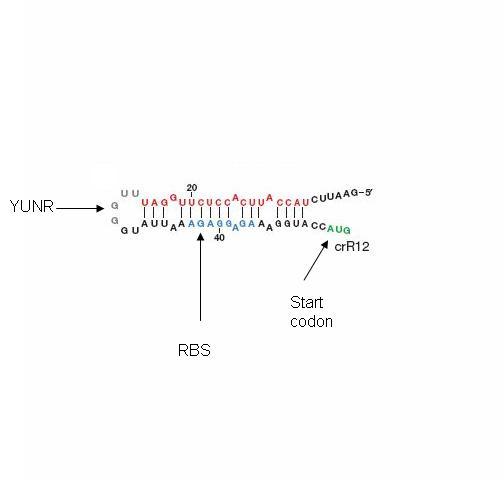
The design itself has two components: a short cis-repressed RNA sequence (crRNA) that is inserted upstream of the gene and a transactivating RNA sequence (taRNA) that targets the crRNA and is not attached to the target. The crRNA sequence contains two fundamental components: the complement of the ribosomal binding site (RBS) and a pyrimidine-uracil-nucleotide-purine (YUNR) sequence (figure 9). When not interacting with the taRNA, the complement of the RBS binds to the RBS, causing the crRNA to loop and block the ribosome's access to the RBS. When the RBS is blocked, translation does not occur; the gene expression is off. The YUNR sequence has a complement on the taRNA. When the taRNA finds a crRNA, the interaction with the YUNR sequence begins pulling the crRNA off the RBS.
(Isaacs et al. 2004)
Figure 9: Cis repressing RNA, cr-RNA, sequence is inserted upstream of the RBS. Part of the cr-RNA (red) complements the RBS and a few bases on either side. This section of the cr-RNA is not an exact complement; thus, the cr-RNA can be peeled off the RBS by the trans activating RNA, ta-RNA. The complementary sequence to YUNR is found in the ta-RNA and begins peeling off the cr-RNA by binding to it (figure 11).
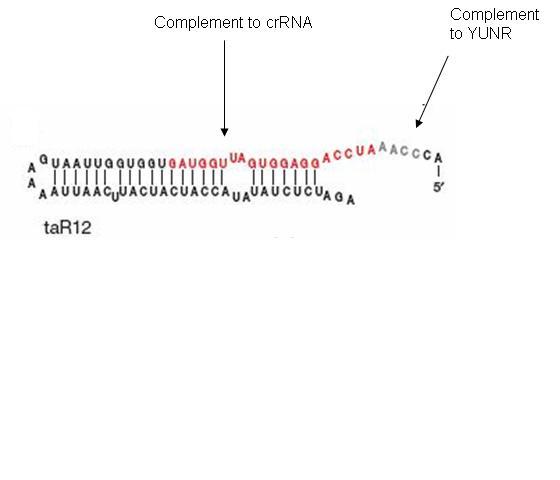
The taRNA is free floating and regulated by an inducible promoter. The inducible promoter allows the researcher to determine under what conditions translation will be allowed. Besides the complement to the YUNR sequence, the taRNA contains a section of high complementarity to section of the crRNA that folds over to cover the RBS, which allows the taRNA to keep the crRNA sequestered from the RBS (figure 10).
(Isaacs et al. 2004)
Figure 10: ta-RNA when not in contact with cr-RNA sequesters the complement to the cr-RNA as it is extremely close to the RBS sequence. When the YUNR complement binds to the YUNR sequence in the cr-RNA, the binding of the cr-RNA to the rest of its complement in the ta-RNA begins, and the cr-RNA is pulled off the RBS (figure 11).
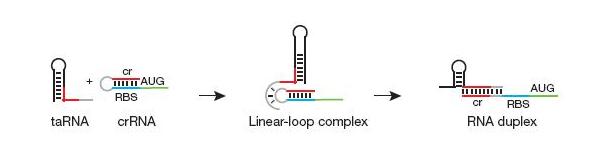
To recap, gene expression is off when there is crRNA upstream of the gene but no taRNA is in the system. In the presence of taRNA, gene expression is turned back on.
(Isaacs et al. 2004)
Figure 11: In a. the cr-RNA is bound to the RBS and blocks ribosomes from translating the mRNA into protein. In b. the ta-RNA comes in contact with the YUNR sequence on the cr-RNA and begins to bind to the rest of the cr-RNA. In c. the ta-RNA fully bound to the cr-RNA and peeled the cr-RNA off the RBS. The RBS is now free for the ribosome to bind to and begin translation.
From Concept to Wet Lab
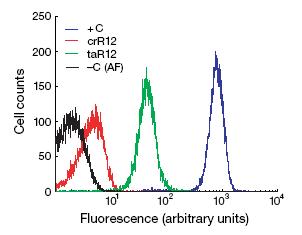
In wet lab, the crRNA sequence was placed in front of the GFP gene and introduced to cells. Using flow cytometry, Isaacs et al. measured fluorescence of cells that had just the GFP gene, the GFP gene with crRNA upstream, the GFP gene with crRNA upstream and taRNA, and no GFP at all. The crRNA decreased fluorescence to near basal levels. When taRNA was present in the system, fluorescence increased by approximately a power of ten. While the fluorescence did not equal fluorescence of cells with just GFP, the repression of gene expression with crRNA and return of expression with taRNA are strong enough to suggest the riboregulator system works.
(Isaacs et al. 2004)
Figure 12: The black curve represents fluoresnce in cells that do not have a GFP gene (ie. the cells natural autofluoresence). The red curve represents fluoresence in cells that have GFP with crRNA upstream. The green curve represents fluoresence in cells that have GFP with crRNA upstream and produce the taRNA. The blue curve represents the fluorescence of cells that have normal GFP minus the cells' autofluoresence.
These experiments were done with pBad and pLac controlling the production of taRNA. As the system worked when the taRNA was under control of either promoter and the crRNA can be inserted upstream of any gene, this system is considered modular.
Finally, riboregulators can control different genes in response to different stinuli by using different crRNA/taRNA pairs as the pairs are specific to each other.
(Isaacs et al. 2004)
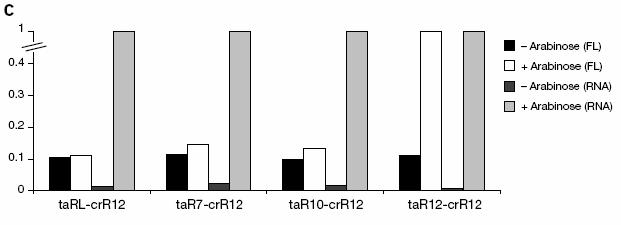
Figure 13: The graph shows both GFP fluorescence (black and white bars) when the ta-RNA promoter, pBad, is off (- arabinose) and on (+ arabinose). All data is normalized to + arabinose GFP an RNA levels. The low level GFP fluorescence when no arabinose is present shows that the efficiency of cr-RNA is not 100% but is still high. As high GFP fluorescence is seen only when high amounts of the matching ta-RNA is present and not simply when any ta-RNA variant is present, this data shows that riboregulator pairs are specific and multiple pairs can be used to regulate multiple genes without fear that any ta-RNA produced would activated all the RNAs and not just its target RNA.
Further Work
As mentioned in the overview, using regulatory proteins or inducible promoters limits the number of stimuli (molecules) that can be used to determine when expression should occur. Generating taRNA that is ligand controlled like an antiswitch or riboswitch would provide a greater versatility for riboregulator use. Ligand controlled riboregulators may also be more effective as control by a ligand may decrease leaky activity, which occurs with promoters as they always allow for some basal level of transcription. Isaacs has continued work to engineer a ligand controlled version of the riboregulator.
References
Isaacs FJ, et al. Engineered riboregulators enable post-transcriptional control of gene expression. Nat Biotechnol. (2004) 22:841-47.