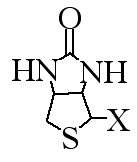
Biotin (also known as vitamin H) is a small organic molecule found in every cell (figure 1). Avidin (also called strepavidin) is a much larger protein that binds biotin with a very high affinity (figure 2). When these two molecules are in the same solution, they will bind with such high affinity that the binding is essentially irreversible. No matter how many times you wash them, they will not let go of each other.
Figure 1. Structure of biotin. Because biotin can be modified differently in different organisms, the X is used to denote a variable side chain.
Figure 2. 3D image avidin (shown as blue and green dimer) from hen egg white bound with biotin (red) as determined by X-ray chrystalography. PDB # 1AVD.
Because of their high affinity, investigators use biotin to tag a molecule of interest and avidin to extract the biotin-tagged protein. For example, the proteomics ICAT method (Proteomics Chapter) uses a reagent that contains biotin (figure 3). Avidin is used to extract biotin/ICAT-tagged peptides to minimize the complexity of the protein mixture for subsequent tandem MS analysis.
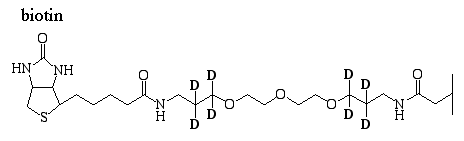
Figure 3. ICAT labeling reagent.
Other uses of biotin and avidin include the labeling of proteins in particular cellular locations. For example, if you could add biotin (biotinolate is the verb) to all proteins on the outside of a cell and then use avidin to purify (via affinity chromotography) these proteins away from all the others in the proteome.