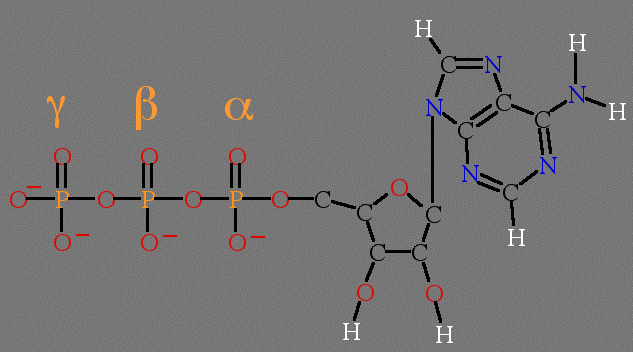

Figure 1. Structure of ATP. Top, line drawing with labeled parts. Bottom panel is an interactive chime image.
The addition of a phosphate covalently modulates the substrate protein and typically alters the substrate conformation sufficiently to either activate or inactivate it. There is no way of knowing in advance whether a kinase will activate or inactivate its substrate. Some kinases can phosphorylate more than one protein and sometimes one substrate will be activated while another will be inactivate (see Complex Genomic Circuits Chapter 8 for examples).
In the Proteomics Chapter 6, Mike Snyder used protein microwell arrays to determine the specificity of almost all of the kinases in the yeast genome. In Snyder's experiments, radioactive phosphorous (33P) was incorporated into the gamma phosphate so when a substrate was phosphorylated, the radioactivity was transferred to the substrate. The amount of 33P transferred to the substrate over time would indicate the rate of activity for each kinase.
Any enzyme can be measured in an assay where either the production of product or the consumption of substrate can be measured. When the accumulation of product or loss of substrate is measured over time and there are no limiting factors (such as very little substrate), then we expect to see the rate of enzyme activit to be linear over time (figure 2). The linearity of single molecule enzyme kinetics was demonstrated by Norm Dovichi in the proteomics chapter.
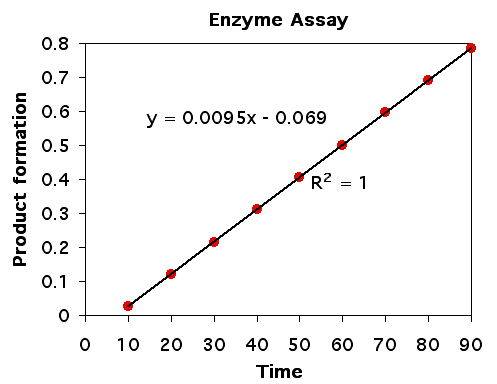
Figure 2. Rate of product formation is linear over time when substrate is plentiful and product accumulation does not inhibit the enzyme.