Difference between revisions of "Summer 2012 SynBio Project (Davidson and MWSU)"
(→GGA for College Teaching Labs) |
Macampbell (talk | contribs) (→Figures for Todd) |
||
| (55 intermediate revisions by 6 users not shown) | |||
| Line 6: | Line 6: | ||
# [[Golden Gate]]<br> | # [[Golden Gate]]<br> | ||
# [[Philosophy and Ethics of our Project]]<br> | # [[Philosophy and Ethics of our Project]]<br> | ||
| + | # [[Summer 2012 Outcomes]]<br> | ||
| Line 244: | Line 245: | ||
*[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1858662/ '''Screen for synthetic riboswitches reveals mechanistic insights into their function'''] | *[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1858662/ '''Screen for synthetic riboswitches reveals mechanistic insights into their function'''] | ||
*[http://www.nature.com/scitable/topicpage/riboswitches-a-common-rna-regulatory-element-14262702 '''Riboswitches: A Common RNA Regulatory Element'''] | *[http://www.nature.com/scitable/topicpage/riboswitches-a-common-rna-regulatory-element-14262702 '''Riboswitches: A Common RNA Regulatory Element'''] | ||
| + | [[File:Theophylline.png]]<br> | ||
| + | '''Figure 1''' After constructing two different riboswitches that are supposed to respond to Theophylline and connecting them to superfolder GFP ([http://partsregistry.org/Part:BBa_J100079 Part J100079] and [http://partsregistry.org/Part:BBa_J100080 Part J100080]), I tested these riboswitch constructs. This figure shows that Riboswitch D worked. In the absence of theophylline, there was very little GFP production, but with increased concentrations of theophylline, the production of GFP increased. Riboswitch E, however, did not work as expected; the theophylline did not permit translation of GFP. This was probably due to a mutation in either the riboswitch or the GFP coding sequence. | ||
| + | |||
| + | [[File:Theophylline and Caffeine.png]]<br> | ||
| + | '''Figure 2''' Although caffeine and theophylline have very similar chemical structures, the Riboswitch D is specific enough to only recognize theophylline. In the presence of increasing concentration of theophylline, Riboswitch D increased the production of GFP. However with the same concentrations of caffeine, the production of GFP did not increase. | ||
| + | |||
| + | List of evolved proteins that can convert caffeine into theoph. From Michener and Smolke. <br> | ||
| + | [[Media:Full_series.pdf]] | ||
==Gas-Phase Communication== | ==Gas-Phase Communication== | ||
| Line 270: | Line 279: | ||
*[http://jb.asm.org/content/186/7/2085.full.pdf '''The Effects of D-lactate on ArcB in Aerobic and Anaerobic Conditions'''] | *[http://jb.asm.org/content/186/7/2085.full.pdf '''The Effects of D-lactate on ArcB in Aerobic and Anaerobic Conditions'''] | ||
*[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC95567/ '''Intracellular Concentration of Hydrogen Peroxide and Catalase in E. coli'''] | *[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC95567/ '''Intracellular Concentration of Hydrogen Peroxide and Catalase in E. coli'''] | ||
| + | |||
| + | So far I have assembled short and long sequences of the fadB promoter (repressed by the ArcAB system) with oligos and have ligated them into an empty plasmid and a plasmid containing RBS+RFP (part K093005) for a total of 4 new parts. I have also done preliminary experiments with DTT and plan to continue to run experiments with DTT and hydrogen peroxide to see how they effect the expression of RFP. | ||
| + | |||
| + | The new part numbers: | ||
| + | J100067 (fadB long promoter) | ||
| + | J100068 (fadB short promoter) | ||
| + | J100077 (J100068:K093005, fadB short+RBS+RFP) | ||
| + | J100078 (J100067:K093005, fadB long+RBS+RFP) | ||
| + | |||
| + | For the preliminary DTT experiment, I grew two tubes of J100077 cells (which had a red tint when grown on a plate). One of these tubes contained 5mM DTT and the other tube had no DTT. I did the same thing with J100078 cells even though they had no visually perceptible red tint. | ||
| + | |||
| + | Results:<br> | ||
| + | [[Image:DTT graph.jpg]]<br> | ||
| + | |||
| + | 2 mL of cells per tube were grown overnight. The tubes labeled +DTT contain 5mM DTT. I measured fluorescence and absorbance with the synergy machine and got the values shown by dividing fluorescence by absorbance. | ||
==Light== | ==Light== | ||
| Line 292: | Line 316: | ||
| '''type of protein'''|| halorhodopsin || n/a || halorhodopsin || BR=bacteriorhodopsin, BO=bacterio-opsin || proteorhodopsin | | '''type of protein'''|| halorhodopsin || n/a || halorhodopsin || BR=bacteriorhodopsin, BO=bacterio-opsin || proteorhodopsin | ||
|- | |- | ||
| − | | '''direction''' || into cell || n/a || into cell || | + | | '''direction''' || into cell || n/a || into cell || out of cell || out of cell |
|} | |} | ||
*pH inducible promoters | *pH inducible promoters | ||
| Line 421: | Line 445: | ||
*[http://www.ncbi.nlm.nih.gov/books/NBK84445/ '''Workshop Summary of Applications of Synthetic Biology'''] | *[http://www.ncbi.nlm.nih.gov/books/NBK84445/ '''Workshop Summary of Applications of Synthetic Biology'''] | ||
| − | + | '''Arrow Diagram Files''' | |
| + | *[[File:ADs_246.zip]] | ||
== GGA for College Teaching Labs== | == GGA for College Teaching Labs== | ||
| Line 428: | Line 453: | ||
'''Objective:''' | '''Objective:''' | ||
| − | The goal is for | + | The goal is for research students to be able to ligate a promoter into the J119044 plasmid with the least hands-on time as possible. If it is successful, the cells transformed from the Golden Gate Assembly (GGA) ligations will show RFP. (see GGA protocol and what goes into ligation [http://gcat.davidson.edu/mediawiki-1.15.0/index.php/Golden_Gate_Assembly_protocol here])'''*''' |
'''Process:''' | '''Process:''' | ||
| − | Previously, I had seen red fluorescence | + | Previously, I had seen red fluorescence on the plate of cells that were transformed with the ligation of J119022 (022) and J119044 (044) plasmids. This ligation was in the PCR machine for 30 cycles, so Dr. Campbell and I decided to repeat the process, but with 5 and 10 cycles in the PCR machine instead. We also tried putting a GGA ligation with cleaned PCR product (J23100 insert)(protocol for cleaning PCR product [http://www.bio.davidson.edu/courses/Molbio/Protocols/Clean_Concentrate.html here]), as opposed to the entire 022 plasmid, in the PCR machine for 5 or 10 cycles. This left us with four experimental plates and two negative control plates (with cells transformed from ligations without enzymes; one in the PCR machine for 5 cycles, one for 10 cycles). |
'''Results:''' | '''Results:''' | ||
| − | After letting the plates incubate overnight, we saw the results. Initially, only the 5 | + | After letting the plates (with cells transformed from the experimental ligations) incubate overnight, we saw the results. Initially, only the plates with cells transformed from the ligations with 022 plasmids (from both 5 and 10 cycles in the PCR machine) had red colonies. However, as the day went on, the plates with cells transformed from the ligations with clean PCR product showed fluorescence, too. The plates sat out for the weekend, and by Tuesday morning, three of the four experimental plates were obviously red. These three were: the plate with cells transformed from the ligation with 022 plasmids that was in the PCR machine for 10 cycles, the plate with cells transformed from the ligation with clean PCR product that was in the PCR machine for 10 cycles, and the plate with cells transformed from the ligation with clean PCR product that was in the PCR machine for 5 cycles. The plate with cells transformed from the ligation with 022 plasmids that was in the PCR machine for 5 cycles had a few red colonies, but not nearly as many as the other three. We took pictures under UV light (seen below) of the three that showed fluorescence plus a negative control plate (with cells that were transformed from a ligation with no enzymes that was in the PCR machine for 10 cycles) as a comparison. The plate with cells transformed from the ligation with clean PCR product that was in the PCR machine for 5 cycles displays the least amount of RFP of the three plates shown, with roughly half of the colonies glowing red. |
These inserts ([http://partsregistry.org/Part:BBa_J119022 J23100]) were cloned into the plasmid [http://partsregistry.org/Part:BBa_J119044 J119044].<br> | These inserts ([http://partsregistry.org/Part:BBa_J119022 J23100]) were cloned into the plasmid [http://partsregistry.org/Part:BBa_J119044 J119044].<br> | ||
<center> | <center> | ||
[[File:4plates.jpeg]]<br> | [[File:4plates.jpeg]]<br> | ||
| − | '''Figure 1.''' Photograph of 4 plates containing transformed ''E. coli'' (JM109) used in this GGA experiment. Clockwise from the top: Negative control, 10-cycle 022 plasmid, 10-cycle PCR product, 5-cycle PCR product | + | '''Figure 1.''' Photograph of 4 plates containing transformed ''E. coli'' (JM109) used in this GGA experiment. Clockwise from the top: Negative control, 10-cycle 022 plasmid ligation, 10-cycle clean PCR product ligation, 5-cycle clean PCR product ligation |
<br> | <br> | ||
[[File:negcontrol.jpeg]]<br> | [[File:negcontrol.jpeg]]<br> | ||
| Line 445: | Line 470: | ||
<br> | <br> | ||
[[File:plasmid10.jpeg]]<br> | [[File:plasmid10.jpeg]]<br> | ||
| − | '''Figure 3.''' Photograph of the 10 | + | '''Figure 3.''' Photograph of the plate with cells transformed from the ligation with 022 plasmids; 10 PCR cycles<br> |
<br> | <br> | ||
[[File:PCR10.jpeg]]<br> | [[File:PCR10.jpeg]]<br> | ||
| − | '''Figure 4.''' Photograph of the 10 | + | '''Figure 4.''' Photograph of the plate with cells transformed from the ligation with clean PCR product; 10 PCR cycles<br> |
<br> | <br> | ||
[[File:PCR5.jpeg]]<br> | [[File:PCR5.jpeg]]<br> | ||
| − | '''Figure 5.''' Photograph of the 5 | + | '''Figure 5.''' Photograph of the plate with cells transformed from the ligation with clean PCR product; 5 PCR cycles<br> |
</center> | </center> | ||
<br> | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | '''*Note:''' The ligation mixture on the GGA protocol does not include the insert that is ligated into the J119044 plasmid. I added however many µL of clean PCR product that was needed to make a concentration of 44 ng. Then, I added however many µL of water that was needed to make a ligation that was 10 µL total. Also note that in the next experiment I raise the total ligation volume to 25 µL because many times I add more than 10 µL of insert. Here, I also added however many µL of water I needed to make a ligation that was 25 µL total. | ||
| + | |||
<br> | <br> | ||
<br> | <br> | ||
| Line 461: | Line 492: | ||
'''Objective:''' | '''Objective:''' | ||
| − | The goal of this experiment was to see if | + | The goal of this experiment was to see if PCR product that has not been cleaned and concentrated (raw PCR product) works as efficiently in Golden Gate Assembly (GGA) as the clean PCR product. If raw PCR product does work as efficiently as clean PCR product in GGA, this would eliminate the step of cleaning the PCR product, thereby making GGA a quicker process. |
'''Process:''' | '''Process:''' | ||
| − | I had eight experimental plates and three control plates. For the experimental plates, I made the GGA | + | I had eight experimental plates and three control plates. For the experimental plates, I made the GGA ligation with different volumes of raw PCR product instead of the J119022 plasmid. I used 1, 5, 10, and 20 µL of raw PCR product, and one of each ligation went through either 5 or 10 cycles in the PCR machine. |
| − | I | + | I compared these experimental plates to the positive controls (plates with cells transformed from the GGA ligation containing 44 ng of clean PCR product; I know from a previous experiment that this should produce RFP) to see what volume (if any) of raw PCR product would produce similar results to the clean PCR product. In the set of plates with cells that were transformed from ligations that were in the PCR machine for 10 cycles, there were two positive controls (one ligation was 25 µL total, and the other was 10 µL; this was to see if adding more water would affect the results). |
| − | I only had one positive control in the 5 | + | I only had one positive control in the set of ligations that was in the PCR machine for 5 cycles because there was not enough clean PCR product to have two. I chose to keep the control ligation that was 10 µL total because I knew that it should definitely work (because of a previous successful experiment), and I didn’t know yet if a 25 µL ligation would produce RFP (the experiment with ligations that were in the PCR machine for 5 cycles was performed a day earlier than that of the ligations that were in the PCR machine for 10 cycles). |
| − | There was | + | There were two negative controls (one was a plate with cells transformed from a ligation that was in the PCR machine for 5 cycles, and the other identical, it was just in the PCR machine for 10 cycles). The negative controls were the same ligations as the experimental ones, just without the insert (PCR product—clean or raw). |
'''Results:''' | '''Results:''' | ||
| − | The two | + | The positive control plates with cells that were transformed from ligations that were in the PCR machine for 10 cycles (with different amounts of water) produced the same amount of colonies (and also the same percentage of red colonies), so '''I can conclude that the amount of water in the solutions does not affect the effectiveness of GGA.''' |
| + | |||
| + | These two positive control plates (with cells that were transformed from the ligations that were in the PCR machine for 10 cycles; 25 µL and 10 µL) both had about 50% of their colonies glowing (see Figures 7 and 8). The control plate containing cells that were transformed from a ligation that was in the PCR machine for 5 cycles, however, had a lawn of colonies in the middle (that did not exhibit RFP) and had red colonies on the periphery of the plate (see Figure 6). This was confusing because last week I performed an identical experiment, and red colonies were across the entire plate, not just the periphery. I’m assuming this is a result of faulty spreading of the cells, because the colonies along the edge of the plate show a significant amount of RFP, indicating that GGA was successful. | ||
| + | |||
| + | As for the experimental plates, only the plates containing cells transformed from the ligations that contained 1 µL of raw PCR product showed RFP, with the plate of the cells that were transformed from this ligation (with 1 µL raw PCR product) and was in the PCR machine for 10 cycles showing even more RFP (see Figures 9 and 10). The plates with cells transformed from the ligations containing 5, 10, and 20 µL of raw PCR product (Figures 11-16) did not exhibit RFP at all, regardless of whether the ligations the cells were transformed with were in the PCR machine for 5 or 10 cycles. In fact, the amount of colonies on the plates had a negative correlation with how much raw PCR product was added to the ligations. | ||
| − | + | I assume that, because the positive controls are already glowing so brightly by this point, all of the plates would have exhibited some red by now if they were going to. Thus, the plates that don’t have any GFP (the negative controls and the plates with cells transformed from ligations containing 5, 10 or 20 µL of raw PCR product) probably will not begin to glow if they have not already. Because the plates with cells transformed from ligations containing 1 µL of raw PCR product have shown RFP, I decided to go forward with these. | |
| − | I plan to | + | '''Next:''' |
| + | I plan to transform ligations identical to those I transformed previously (containing 1 µL of raw PCR product), except they will be in the PCR machine for 20 or 30 cycles rather than 5 or 10. Because the plate with cells transformed from the ligation containing 1 µL raw PCR product that was in the PCR machine for 10 cycles had a higher percentage of colonies exhibiting RFP than the plate with cells transformed from an identical ligation that was in the PCR machine for 5 cycles, I am guessing that the more cycles the solution goes through, the higher percentage of colonies on the plate will exhibit RFP. I will be transforming four experimental ligations: two will be in the PCR machine for 20 cycles, and two for 30 cycles. Two will have a total volume of 10 µL and two will have a total volume of 25µL (one of each will be in the PCR machine for either 20 or 30 cycles). I am testing two different volumes because I previously got positive results with a total of 25 µL, and I want to verify that the amount of water in the ligation doesn't affect the success of the GGA ligation containing 1 µL raw PCR product (just as I found it doesn't affect the success of the GGA ligation containing 44 ng clean PCR product). | ||
| − | If | + | If putting the ligations in the PCR machine for more cycles does turn out to produce higher percentages of red fluorescent colonies in plates, then professors can have options of how to perform GGA in their classroom. They can choose to add the extra step of cleaning the PCR product (and then only have to put the ligation in for 5 cycles in the PCR machine), or they can not clean the PCR product and put the ligation in the PCR machine for more time (if this ends up being a viable option; I will find out soon). |
| + | <br> | ||
These inserts ([http://partsregistry.org/Part:BBa_J119022 J23100]) were cloned into the plasmid [http://partsregistry.org/Part:BBa_J119044 J119044]. | These inserts ([http://partsregistry.org/Part:BBa_J119022 J23100]) were cloned into the plasmid [http://partsregistry.org/Part:BBa_J119044 J119044]. | ||
<br> | <br> | ||
<center> | <center> | ||
[[File:poscontrol5cycles.jpg|400px|]]<br> | [[File:poscontrol5cycles.jpg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 6:''' Photograph of a plate containing transformed ''E. coli'' (JM109) used in this GGA experiment; Positive control plate with cells transformed from the ligation (10 µL total) that contained 44 ng clean PCR product; 5 PCR cycles; lawn of cells is in the middle; RFP visible on periphery of plate where individual colonies can be seen.<br> |
<br> | <br> | ||
[[File:10µLposcontrol10cycles.jpg|400px|]]<br> | [[File:10µLposcontrol10cycles.jpg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 7:''' Photograph of positive control plate with cells transformed from ligation (10 µL total) containing clean PCR product; 10 PCR cycles<br> |
<br> | <br> | ||
[[File:25µLposcontrol10cycles.jpg|400px|]]<br> | [[File:25µLposcontrol10cycles.jpg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 8:''' Photograph of positive control plate with cells transformed from ligation (25 µL total) containing clean PCR product; 10 PCR cycles<br> |
<br> | <br> | ||
| − | [[File: | + | [[File:Screen shot 2012-07-02 at 10.37.49 AM.png|400px|]]<br> |
| − | '''Figure | + | '''Figure 9:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 1 µL raw PCR product; 5 PCR cycles; circles indicate some locations of colonies exhibiting RFP<br> |
<br> | <br> | ||
[[File:1µL10cycles.jpg|400px|]]<br> | [[File:1µL10cycles.jpg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 10:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 1 µL raw PCR product, 10 PCR cycles<br> |
<br> | <br> | ||
[[File:5µL5cycles.jpeg|400px|]]<br> | [[File:5µL5cycles.jpeg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 11:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 5 µL raw PCR product, 5 PCR cycles<br> |
<br> | <br> | ||
[[File:5µL10cycles.jpeg|400px|]]<br> | [[File:5µL10cycles.jpeg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 12:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 5 µL raw PCR product, 10 PCR cycles<br> |
<br> | <br> | ||
[[File:10µL5cycles.jpeg|400px]]<br> | [[File:10µL5cycles.jpeg|400px]]<br> | ||
| − | '''Figure | + | '''Figure 13:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 10 µL raw PCR product, 5 PCR cycles<br> |
<br> | <br> | ||
[[File:10µL10cycles.jpeg|400px|]]<br> | [[File:10µL10cycles.jpeg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 14:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 10 µL raw PCR product, 10 PCR cycles<br> |
<br> | <br> | ||
[[File:20µL5cycles.jpeg|400px|]]<br> | [[File:20µL5cycles.jpeg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 15:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 20 µL raw PCR product, 5 PCR cycles<br> |
<br> | <br> | ||
[[File:20µL10cycles.jpeg|400px|]]<br> | [[File:20µL10cycles.jpeg|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 16:''' Photograph of plate with cells transformed from ligation (25 µL total) containing 20 µL raw PCR product, 10 PCR cycles<br> |
<br> | <br> | ||
[[File:negcontrol5cycles.JPG|400px|]]<br> | [[File:negcontrol5cycles.JPG|400px|]]<br> | ||
| − | '''Figure | + | '''Figure 17:''' Photograph of negative control plate with cells transformed from ligation (25 µL total) with no insert, 5 PCR cycles<br> |
<br> | <br> | ||
| + | [[File:negcontrol10cycles.JPG|400px|]]<br> | ||
| + | '''Figure 18:''' Photograph of negative control plate with cells transformed from ligation (25 µL total) with no insert, 10 PCR cycles<br> | ||
| + | <br><br><br> | ||
| + | </center> | ||
| + | '''*Note:''' Up to this point, I used only 1 µL of the 10X Promega ligase buffer in every ligation. I should have used 2.5 µL of this buffer in the ligations that were 25 µL total. This could be a possible reason for GGA being unsuccessful in the plates with cells that were transformed from 25 µL ligations. | ||
| + | |||
| + | ==Figures for Todd== | ||
| + | |||
| + | [[File:Fire-exit-horiz.jpg|200px|]] | ||
| + | |||
| + | |||
| + | [[File:123_andy.jpg|200px|]] | ||
| + | |||
| + | |||
| + | [[File:Chambers.png|200px|]] | ||
| + | |||
| + | |||
| + | [[File:sfGFP.jpg|200px|]] | ||
| + | |||
| + | |||
| + | [[File:EMcoli.jpg|200px|]] | ||
| + | |||
| + | |||
| + | [[File:CarolineVrana.jpg|200px|]] | ||
| + | |||
| + | |||
| + | [[File:AnnieWacker.JPG|200px|]] | ||
Latest revision as of 21:37, 2 August 2012
- Davidson Protocols
- MWSU_protocols
- GCAT-alog Freezer Stocks
- Laboratory_Notebooks
- Golden Gate
- Philosophy and Ethics of our Project
- Summer 2012 Outcomes
Contents
Student Proposals from Ind. Studies
- Erich Baker Proposal: Media:Erich_Baker_proposal.docx This proposal deals with Phytochromes and Light Sensitive Channel Proteins
-I think the use of Phytochromes might be a good way to have either a continual stimulus that would repress/express certain genes that could be turned off and on depending on what we want them to do. There are other aspects of the research in this proposal that if not used outright, could be adapted to our continuing projects as either controls or feedback mechanisms. As for the proposed Salis RBS sites, I would like to see more information in the efficacy of the predicted RBS sequence. Possibly if we could use some of the C-Dog information based on a few known sequences to determine if the computer can predict those RBS's we know to be effective then we might be able to count on the calculator as a tool for our experimental design. -Caleb Carr
- Ben Clarkson Proposal: Media:Ben_Clarkson_proposal.docx
- Duke DeLoache Proposal: Media:Duke_DeLoache_Proposal.docx
- Becca Evans Proposal: Media:Becca_Evans_proposal.docx
- Ellen Johnson Proposal: Media:Ellen_Johnson_proposal.docx
PPT Presentations
- This PPT file contains all the slides from student presentations addressing the idea proposed by MWSU.
Media:Reports_on_Circuits.pptx
- This PPT contains slides summarizing some of the best and most complicated papers from Week 11.
Papers
Methods Papers
- DNA assembly for synthetic biology: from parts to pathways and beyond
Tom Ellis, Tom Adieac and Geoff S. Baldwin
Integr. Biol., 2011, 3, 109–118
- Everyone should watch this 5 minute video on optogenetics. Combine that video with the 2010 champoinship iGEM invention of E. glowi.
Older Lab Papers
- Engineering bacteria to solve the Burnt Pancake Problem.
Haynes, Karmella, et al.
Journal of Biological Engineering. Vol. 2(8): 1 – 12.
- Solving a Hamiltonian Path Problem with a Bacterial Computer.
Baumgardner, Jordan et al.
Journal of Biological Engineering. Vol. 3:11
- Bacterial Hash Function Using DNA-Based XOR Logic Reveals Unexpected Behavior of the LuxR Promoter.
Brianna Pearson*, Kin H. Lau* et al.
Interdisciplinary Bio Central. Vol. 3, article no. 10.
Time Delayed Growth Movie
Network Papers
Jonathan M. Raser and Erin K. O’Shea
Science. Vol. 309, page 2010
Please post pdf.
Nagarajan Nandagopal and Michael B. Elowitz
Science. Vol. 333, page 1244.
Please post pdf.
R. Milo, S. Shen-Orr, et al
Science. Vol. 298, page 824.
Please post pdf.
Yang-Yu Liu, Jean-Jacques Slotine, & Albert-La ́szlo ́ Baraba ́si
Nature. 2011. Vol. 473, page 167.
Please post pdf.
Ethics Papers
Colin Mcilswain
Nature. Vol 465, page 867.
- Word selection affects perceptions of synthetic biology. Brianna Pearson, Sam Snell, Kyri Bye-Nagel, Scott Tonidandel, Laurie J Heyer, and A Malcolm Campbell.
-This paper does a great job at highlighting the importance of socio-political legitimation in the funding of science. It seems that all new sciences must survive a period during which their only funding comes from public sources under the condition that those conducting it can make some kind of promises of future benefit to the society as a whole. After proving itself not only useful but also profitable, private money may then start flowing in, though by that point, the nature of that field may arguably have changed for better or worse. I think we would all agree that synthetic biology holds more promise than we can currently even imagine, both for advancing the public good and for providing opportunity for profit (in more than just pharmaceuticals), but it's not enough for us to believe it. Those of us who will someday pursue grants and/or private investments in synthetic biology must learn to speak not only the rational language of the science of synthetic biology but also the politically-driven language of the social benefits of synthetic biology, the socially conscious language of the ethics of synthetic biology, and the profit-driven language of the (future) business of synthetic biology (and possibly others). -Eddie Miles
- Read "Moral" ethics paper on synthetic biology. Media:Moral.pdf
- Read "Future" ethics paper on synthetic biology. Media:Future.pdf
Questions to Consider About Network Pathways
- Are they naturally occurring or synthetic?
- Do they involve screening or selection?
- Are they anabolic or catabolic?
- How many steps are in each pathway?
- How can they relate to cell fitness?
- What specific challenges would need to be addressed if we worked with the pathway?
Cellular Automata
- [1] General CA introduction
- [2], [3] Elementary Cellular Automata
- [4] Good explanation of how elementary CAs work
- [5] Rule 110
- iGEM Team Groningen
- iGEM Team IPN-UNAM Mexico
- MIT 2011 iGEM Tissue Design
- In Vivo Cellular Automata
- Edge Detection PDF
- Patterning of E. coli
- Tunable Bacterial Band-Pass Filter
- E. coli Predator-Prey Ecosystem
- Multicellular System for Programmed Pattern Formation
Peptides
- Pep-1 can carry large amounts of cargo across cell membrane
- Pep-1 has no anti-microbial activity against E. coli, see page 121
- [6]General Manual for CPP After opening, click on the PDF General Manual for detailed information concerning Cellular Permeating Peptides, and products of the like.
- General info on Pep-1
- Very clear, easy to read, discussion on how CPPs work, and more specific info on Pep-1, look in Chapter 1 to start
- Pep-1 is a synthetic peptide
- Pep-1 fusion protein made in E. coli
- Targeting proteins to E. coli periplasmic space (GFP)
- Review of targeting proteins to periplasm
Environmental factors that enhance the action of the cell penetrating peptide pep-1 - A spectroscopic study using lipidic vesicles [[7]]
Assembly
[8]iGEM Introduction to Gibson Assembly
[9]Enzymatic assembly of DNA molecules up to several hundred kilobases
[10] Supplemental Methods for Enzymatic assembly of DNA molecules up to several hundred kilobases
[11]Assembly of BioBricks by the Gibson Method
[12] Properties of Exonuclease
[13]Tool for using Gibson Assembly Method
Library of Parts
Research Papers, Articles & Manuscripts--all inclusive and in regards to any and all parts that are listed, or wish to be listed
- [14] gene-specific promoter element is required for optimal expression of the histone H1 gene in S-phase.
- [15] Multiple Sigma Factors
Promoters Section
- 6 possible promoters for project 3 constitutive, 3 inducible - (Word file not yet saved on wiki)
C-Dog Section
Degradation Tag Section
Selection Modules
Bad-ish genes/proteins
- Toxicity of rat insulin gene on E.coli
- SacB gene with sucrose and E.coli
- Hda-mediated homeostasis in E.coli
- Lon protease from M.smegmatis
- SinI enzyme has moderate growth-inhibition in E.coli
- Excess violecein production toxic to E.coli
- ToxN inhibits growth of E.coli
- Eukaryotic membrane proteins toxic to E.coli
- HbpA proteins moderately toxic to E.coli
- Alpha-luffin and E.coli
- BBG29 gene from 'Borrelia'
Good genes/proteins
CRISPR process
- Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli
- Envelope stress trigger for CRISPR response - and background info on E.coli Cascade complex
- CRISPR database - to compare and find
- CRISPR immune system in Sulfolobales
- CRISPR System protects Microbes against Phages, Plasmids
- Impact on Small Repeat Sequences on Bacterial Genome Evolution
- Structural Basis for CRISPR RNA-guided DNA recognition by Cascade
- Structures of the RNA-guided surveillance complex from a bacterial immune system -figures of Subnanometer structures of Cascade
- 2011 iGEM team- CRISPR/Cas and GFP
- Diversity of CRISPR loci in E.coli
- Guild of 45 CRISPR-associated protein families
- CRISPR Interference Directs Strand Specific Spacer Acquisition
- CRISPR interference: RNA-directed Adaptive Immunity in Bacteria and Archaea
- Mature crRNA length measured by ruler mechanism
- CRISPR-Cas Systems in Bacteria and Archaea: Versatile Small RNAs for Adaptive Defense and Regulation
- Media:CRISPR-based adaptive immune systems-terns.pdf
- Media:Evolution and Classification of the CRISPR-Cas systems- Makarova.pdf
- Media:Essential features and rational design of CRISPR RNAs that function with the Cas RAMP Module complex to cleave RNAs.pdf
- Media:Supplemental information to Essential Features paper.pdf
- The CRISPR System: small RNA-guided defense in bacteria and archaea
- The Escherichia coli CRISPR System Protects from lambda Lysogenization, Lysogens, and Prophage Induction
- Short motif sequences determine the targets of the prokaryotic CRISPR defense system
- The Small, Slow, and Specialized CRISPR and Anti-CRISPR of Escherichia and Salmonella
Regulated Biosynthesis Pathways
http://cat.inist.fr/?aModele=afficheN&cpsidt=6828850
Aptamers
- Use of riboswitch in Bacteria
- Screen for synthetic riboswitches reveals mechanistic insights into their function
- Riboswitches: A Common RNA Regulatory Element
Figure 1 After constructing two different riboswitches that are supposed to respond to Theophylline and connecting them to superfolder GFP (Part J100079 and Part J100080), I tested these riboswitch constructs. This figure shows that Riboswitch D worked. In the absence of theophylline, there was very little GFP production, but with increased concentrations of theophylline, the production of GFP increased. Riboswitch E, however, did not work as expected; the theophylline did not permit translation of GFP. This was probably due to a mutation in either the riboswitch or the GFP coding sequence.
Figure 2 Although caffeine and theophylline have very similar chemical structures, the Riboswitch D is specific enough to only recognize theophylline. In the presence of increasing concentration of theophylline, Riboswitch D increased the production of GFP. However with the same concentrations of caffeine, the production of GFP did not increase.
List of evolved proteins that can convert caffeine into theoph. From Michener and Smolke.
Media:Full_series.pdf
Gas-Phase Communication
- Biopixel Paper
- ArcAB system in V. fischeri Includes promoter sequences
- ArcAB system in E. coli
- ArcAB system Responses to Hydrogen Peroxide in E. coli
- Visual Diagram of ArcAB System in E. coli
- Amino Acid Sequence for ArcA in E. coli
- Amino Acid Sequence for ArcB in E. coli
- Amino Acid Sequence for ArcA in V. Fischeri
- Blast Amino Acid Sequence Comparison for ArcA in E. coli and V. fischeri
- Blast Amino Acid Sequence Comparison for ArcB in E. coli and V. fischeri
- Potential Promoters that ArcA Might Bind To in E. coli
- More Potential Promoters that ArcA Might Bind To in E. coli-fad regulon
- List of Operons Repressed or Activated by ArcA in E. coli
- Sequence of focAP2 promoter-activated by ArcA
- Sequence of cydAP1 promoter-activated by ArcA
- Sequence of icdAp1 promoter-repressed by ArcA
- CydAB Activated in E. coli
- Sub-lethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis
- ArcAB system and how it works-sort of
- Specifics of how ArcB works/its composition
- How ArcAB functions as resistance to reactive oxygen stress/hydrogen peroxide
- ArcAB and the cydAB promoter with the H-NS protein
- The Effects of D-lactate on ArcB in Aerobic and Anaerobic Conditions
- Intracellular Concentration of Hydrogen Peroxide and Catalase in E. coli
So far I have assembled short and long sequences of the fadB promoter (repressed by the ArcAB system) with oligos and have ligated them into an empty plasmid and a plasmid containing RBS+RFP (part K093005) for a total of 4 new parts. I have also done preliminary experiments with DTT and plan to continue to run experiments with DTT and hydrogen peroxide to see how they effect the expression of RFP.
The new part numbers: J100067 (fadB long promoter) J100068 (fadB short promoter) J100077 (J100068:K093005, fadB short+RBS+RFP) J100078 (J100067:K093005, fadB long+RBS+RFP)
For the preliminary DTT experiment, I grew two tubes of J100077 cells (which had a red tint when grown on a plate). One of these tubes contained 5mM DTT and the other tube had no DTT. I did the same thing with J100078 cells even though they had no visually perceptible red tint.
Results:
2 mL of cells per tube were grown overnight. The tubes labeled +DTT contain 5mM DTT. I measured fluorescence and absorbance with the synergy machine and got the values shown by dividing fluorescence by absorbance.
Light
- light-gated ion channels/pumps
| Pump | phR | MscL | NpHR | e-BO/e-BR/h-BR | PR |
|---|---|---|---|---|---|
| wavelength | max absorbance at 578-599 | open with 366 nm, close with visible light (>466 nm) | 578 nm (with NaCl in media) | 550-560 nm | ~525 nm |
| particles that can travel through it | Chloride ions | non-selective, 3-nm diameter | anions | protons | protons |
| pump/channel? | pump | channel | pump | pump | pump |
| type of protein | halorhodopsin | n/a | halorhodopsin | BR=bacteriorhodopsin, BO=bacterio-opsin | proteorhodopsin |
| direction | into cell | n/a | into cell | out of cell | out of cell |
- pH inducible promoters
- pH inducible promoter system pSM10
- Media:pH inducible promoter.pdf pSM-10
- High pH induced proteins
- more high/low pH induced proteins/genes
- high/low pH induced proteins/genes (more)
- TnaA
- cpxP (high ph induced)
- cadA promoter sequence (low pH induced)
- rest of cadA promoter sequence
- Altered pH and lysine signalling mutants of cadC, a gene encoding a membrane-bound transcriptional activator of the Escherichia ecoli cadBA operon
- Identification of elements involved in transcriptional regulation of the Escherichia coli cad operon by external pH.
- File:Küper and Jung 2005.pdf Cad1 and Cad2 positions/sequences in cadBA promoter
- E. coli maintain a relatively constant intracellular pH (pumps sense extracellular pH)
- CysK promoter (high pH)
- luciferin regeneration
Blue Light Regulated Promoter YgcF
- Articles/ References:
- Proposed Pathway:
- Parts to Build:
- K238013
- gnl|ECOLI|G6603
- gnl|ECOLI|G6602
- Additional Parts:
- Vector: PSB1A2 (Isolated and purified from a gel)
- Insert with plac promoter and RFP gene: J04450
Maths
- Networks (Modeling Focused)
- Field might be kind of saturated; it seems like a lot of work has been done.
- But not with netLogo. How could that work?
- RePast: another ABM suite that might be mroe suited to networks [21]
- General
- Network motifs in the transcriptional regulation network of Escherichia coli
- [22] Gene Regulatory Network wikipedia page
- [23] long dissertation on modeling GRNs
- Large-Scale Mapping and Validation of Escherichia coli Transcriptional Regulation from a Compendium of Expression Profiles
- Process Calculus
- [24] Process Calculus wikipedia page
- [BioAmbients: An abstraction for biological compartments Process Calculi for bio modelling; might be at the level of cells as opposed to genes etc.
- Boolean Networks
- [25] Boolean Network wiki page; elementary CA are special cases of Boolean networks
- Boolean modeling of GRNs
- 'The regulatory network of E. coli metabolism as a Boolean dynamical system exhibits both homeostasis and flexibility of response
- Or-Not Logic Gate with E.Coli
- Dynamical Systems
- Discrete dynamical system modelling for gene regulatory networks of 5-hydroxymethylfurfural tolerance for ethanologenic yeast
- A Linear Discrete Dynamic System Model for Temporal Gene Interaction and Regulatory Network Influence in Response to Bioethanol Conversion Inhibitor HMF for Ethanologenic Yeast
- Field might be kind of saturated; it seems like a lot of work has been done.
- Flux Balance Analysis
- Agent Based Models/Complex Adaptive Systems
- Real Computing/Complexity
- [31] Lecture transcripts from two MIT courses on compleity by a very smart guy in the field
- [32] Review of physical computing by the same researcher
- [33] Part of a textbook on computation theory
- [34] Harvard analog computing
- [35] Free draft of Princeton text on computational complexity
- [36] Paper written by one of the authors of Complexity and Real Computation that contains the same basic ideas
- [37] An analog computer museum and information site run by a Dr. Bernd Ulmann, who did his doctoral thesis on analog computing
- [38] Abstract of a 1964 study that used analog computers to model a bacterial cell
- [39] Paper on combined use of analog and digital computation
- [40] First 28 pages of Neural Networks and Analog Computing: Beyond the Turing Limit
- Neural Networks
- Fuzzy Logic/Modeling
- [44] Soft Computing (general field)
- [45] A whole book on fuzzy rule based modeling
- [46] Fuzzy modeling and control of biological processes
- Systems biology by the rules: hybrid intelligent systems for pathway modeling and discovery
- [47] FCL Java package
- [48] Noise-based logic
- [49] Fuzzy sets overview slides (MIT)
Communication
- Bacterial Conjugation
General
Arrow Diagram Files
GGA for College Teaching Labs
6/21/12 Experiment
Objective: The goal is for research students to be able to ligate a promoter into the J119044 plasmid with the least hands-on time as possible. If it is successful, the cells transformed from the Golden Gate Assembly (GGA) ligations will show RFP. (see GGA protocol and what goes into ligation here)*
Process: Previously, I had seen red fluorescence on the plate of cells that were transformed with the ligation of J119022 (022) and J119044 (044) plasmids. This ligation was in the PCR machine for 30 cycles, so Dr. Campbell and I decided to repeat the process, but with 5 and 10 cycles in the PCR machine instead. We also tried putting a GGA ligation with cleaned PCR product (J23100 insert)(protocol for cleaning PCR product here), as opposed to the entire 022 plasmid, in the PCR machine for 5 or 10 cycles. This left us with four experimental plates and two negative control plates (with cells transformed from ligations without enzymes; one in the PCR machine for 5 cycles, one for 10 cycles).
Results: After letting the plates (with cells transformed from the experimental ligations) incubate overnight, we saw the results. Initially, only the plates with cells transformed from the ligations with 022 plasmids (from both 5 and 10 cycles in the PCR machine) had red colonies. However, as the day went on, the plates with cells transformed from the ligations with clean PCR product showed fluorescence, too. The plates sat out for the weekend, and by Tuesday morning, three of the four experimental plates were obviously red. These three were: the plate with cells transformed from the ligation with 022 plasmids that was in the PCR machine for 10 cycles, the plate with cells transformed from the ligation with clean PCR product that was in the PCR machine for 10 cycles, and the plate with cells transformed from the ligation with clean PCR product that was in the PCR machine for 5 cycles. The plate with cells transformed from the ligation with 022 plasmids that was in the PCR machine for 5 cycles had a few red colonies, but not nearly as many as the other three. We took pictures under UV light (seen below) of the three that showed fluorescence plus a negative control plate (with cells that were transformed from a ligation with no enzymes that was in the PCR machine for 10 cycles) as a comparison. The plate with cells transformed from the ligation with clean PCR product that was in the PCR machine for 5 cycles displays the least amount of RFP of the three plates shown, with roughly half of the colonies glowing red.
These inserts (J23100) were cloned into the plasmid J119044.
Figure 1. Photograph of 4 plates containing transformed E. coli (JM109) used in this GGA experiment. Clockwise from the top: Negative control, 10-cycle 022 plasmid ligation, 10-cycle clean PCR product ligation, 5-cycle clean PCR product ligation
Figure 2. Photograph of the negative control plate
Figure 3. Photograph of the plate with cells transformed from the ligation with 022 plasmids; 10 PCR cycles
Figure 4. Photograph of the plate with cells transformed from the ligation with clean PCR product; 10 PCR cycles
Figure 5. Photograph of the plate with cells transformed from the ligation with clean PCR product; 5 PCR cycles
*Note: The ligation mixture on the GGA protocol does not include the insert that is ligated into the J119044 plasmid. I added however many µL of clean PCR product that was needed to make a concentration of 44 ng. Then, I added however many µL of water that was needed to make a ligation that was 10 µL total. Also note that in the next experiment I raise the total ligation volume to 25 µL because many times I add more than 10 µL of insert. Here, I also added however many µL of water I needed to make a ligation that was 25 µL total.
6/28/12 Experiment
Objective: The goal of this experiment was to see if PCR product that has not been cleaned and concentrated (raw PCR product) works as efficiently in Golden Gate Assembly (GGA) as the clean PCR product. If raw PCR product does work as efficiently as clean PCR product in GGA, this would eliminate the step of cleaning the PCR product, thereby making GGA a quicker process.
Process: I had eight experimental plates and three control plates. For the experimental plates, I made the GGA ligation with different volumes of raw PCR product instead of the J119022 plasmid. I used 1, 5, 10, and 20 µL of raw PCR product, and one of each ligation went through either 5 or 10 cycles in the PCR machine.
I compared these experimental plates to the positive controls (plates with cells transformed from the GGA ligation containing 44 ng of clean PCR product; I know from a previous experiment that this should produce RFP) to see what volume (if any) of raw PCR product would produce similar results to the clean PCR product. In the set of plates with cells that were transformed from ligations that were in the PCR machine for 10 cycles, there were two positive controls (one ligation was 25 µL total, and the other was 10 µL; this was to see if adding more water would affect the results).
I only had one positive control in the set of ligations that was in the PCR machine for 5 cycles because there was not enough clean PCR product to have two. I chose to keep the control ligation that was 10 µL total because I knew that it should definitely work (because of a previous successful experiment), and I didn’t know yet if a 25 µL ligation would produce RFP (the experiment with ligations that were in the PCR machine for 5 cycles was performed a day earlier than that of the ligations that were in the PCR machine for 10 cycles).
There were two negative controls (one was a plate with cells transformed from a ligation that was in the PCR machine for 5 cycles, and the other identical, it was just in the PCR machine for 10 cycles). The negative controls were the same ligations as the experimental ones, just without the insert (PCR product—clean or raw).
Results: The positive control plates with cells that were transformed from ligations that were in the PCR machine for 10 cycles (with different amounts of water) produced the same amount of colonies (and also the same percentage of red colonies), so I can conclude that the amount of water in the solutions does not affect the effectiveness of GGA.
These two positive control plates (with cells that were transformed from the ligations that were in the PCR machine for 10 cycles; 25 µL and 10 µL) both had about 50% of their colonies glowing (see Figures 7 and 8). The control plate containing cells that were transformed from a ligation that was in the PCR machine for 5 cycles, however, had a lawn of colonies in the middle (that did not exhibit RFP) and had red colonies on the periphery of the plate (see Figure 6). This was confusing because last week I performed an identical experiment, and red colonies were across the entire plate, not just the periphery. I’m assuming this is a result of faulty spreading of the cells, because the colonies along the edge of the plate show a significant amount of RFP, indicating that GGA was successful.
As for the experimental plates, only the plates containing cells transformed from the ligations that contained 1 µL of raw PCR product showed RFP, with the plate of the cells that were transformed from this ligation (with 1 µL raw PCR product) and was in the PCR machine for 10 cycles showing even more RFP (see Figures 9 and 10). The plates with cells transformed from the ligations containing 5, 10, and 20 µL of raw PCR product (Figures 11-16) did not exhibit RFP at all, regardless of whether the ligations the cells were transformed with were in the PCR machine for 5 or 10 cycles. In fact, the amount of colonies on the plates had a negative correlation with how much raw PCR product was added to the ligations.
I assume that, because the positive controls are already glowing so brightly by this point, all of the plates would have exhibited some red by now if they were going to. Thus, the plates that don’t have any GFP (the negative controls and the plates with cells transformed from ligations containing 5, 10 or 20 µL of raw PCR product) probably will not begin to glow if they have not already. Because the plates with cells transformed from ligations containing 1 µL of raw PCR product have shown RFP, I decided to go forward with these.
Next: I plan to transform ligations identical to those I transformed previously (containing 1 µL of raw PCR product), except they will be in the PCR machine for 20 or 30 cycles rather than 5 or 10. Because the plate with cells transformed from the ligation containing 1 µL raw PCR product that was in the PCR machine for 10 cycles had a higher percentage of colonies exhibiting RFP than the plate with cells transformed from an identical ligation that was in the PCR machine for 5 cycles, I am guessing that the more cycles the solution goes through, the higher percentage of colonies on the plate will exhibit RFP. I will be transforming four experimental ligations: two will be in the PCR machine for 20 cycles, and two for 30 cycles. Two will have a total volume of 10 µL and two will have a total volume of 25µL (one of each will be in the PCR machine for either 20 or 30 cycles). I am testing two different volumes because I previously got positive results with a total of 25 µL, and I want to verify that the amount of water in the ligation doesn't affect the success of the GGA ligation containing 1 µL raw PCR product (just as I found it doesn't affect the success of the GGA ligation containing 44 ng clean PCR product).
If putting the ligations in the PCR machine for more cycles does turn out to produce higher percentages of red fluorescent colonies in plates, then professors can have options of how to perform GGA in their classroom. They can choose to add the extra step of cleaning the PCR product (and then only have to put the ligation in for 5 cycles in the PCR machine), or they can not clean the PCR product and put the ligation in the PCR machine for more time (if this ends up being a viable option; I will find out soon).
These inserts (J23100) were cloned into the plasmid J119044.

Figure 6: Photograph of a plate containing transformed E. coli (JM109) used in this GGA experiment; Positive control plate with cells transformed from the ligation (10 µL total) that contained 44 ng clean PCR product; 5 PCR cycles; lawn of cells is in the middle; RFP visible on periphery of plate where individual colonies can be seen.

Figure 7: Photograph of positive control plate with cells transformed from ligation (10 µL total) containing clean PCR product; 10 PCR cycles

Figure 8: Photograph of positive control plate with cells transformed from ligation (25 µL total) containing clean PCR product; 10 PCR cycles
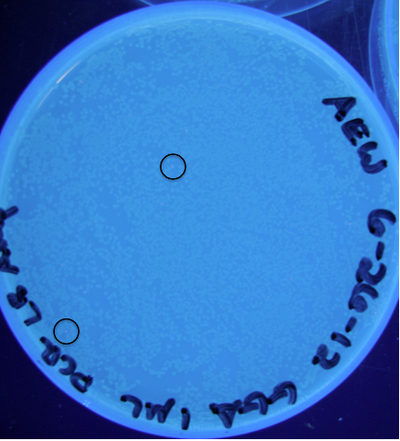
Figure 9: Photograph of plate with cells transformed from ligation (25 µL total) containing 1 µL raw PCR product; 5 PCR cycles; circles indicate some locations of colonies exhibiting RFP

Figure 10: Photograph of plate with cells transformed from ligation (25 µL total) containing 1 µL raw PCR product, 10 PCR cycles

Figure 11: Photograph of plate with cells transformed from ligation (25 µL total) containing 5 µL raw PCR product, 5 PCR cycles

Figure 12: Photograph of plate with cells transformed from ligation (25 µL total) containing 5 µL raw PCR product, 10 PCR cycles

Figure 13: Photograph of plate with cells transformed from ligation (25 µL total) containing 10 µL raw PCR product, 5 PCR cycles

Figure 14: Photograph of plate with cells transformed from ligation (25 µL total) containing 10 µL raw PCR product, 10 PCR cycles

Figure 15: Photograph of plate with cells transformed from ligation (25 µL total) containing 20 µL raw PCR product, 5 PCR cycles

Figure 16: Photograph of plate with cells transformed from ligation (25 µL total) containing 20 µL raw PCR product, 10 PCR cycles
Figure 17: Photograph of negative control plate with cells transformed from ligation (25 µL total) with no insert, 5 PCR cycles
Figure 18: Photograph of negative control plate with cells transformed from ligation (25 µL total) with no insert, 10 PCR cycles
*Note: Up to this point, I used only 1 µL of the 10X Promega ligase buffer in every ligation. I should have used 2.5 µL of this buffer in the ligations that were 25 µL total. This could be a possible reason for GGA being unsuccessful in the plates with cells that were transformed from 25 µL ligations.









